Some Basic Concepts of Chemistry ?
Basic concepts of chemistry form the foundation for understanding how matter behaves, interacts, and changes. Here are some fundamental concepts:
- Matter: Anything that has mass and occupies space. Matter exists in three primary states: solid, liquid, and gas.
- Atoms and Molecules:
- Atoms: The smallest unit of matter that retains the properties of an element. It consists of protons, neutrons, and electrons.
- Molecules: Two or more atoms bonded together. They can be the same type of atoms (like O₂) or different (like H₂O).
- Elements: Pure substances made of only one type of atom. Examples include hydrogen (H), oxygen (O), and carbon (C).
- Compounds: Substances formed when two or more different elements chemically bond. Examples include water (H₂O), sodium chloride (NaCl), and carbon dioxide (CO₂).
- Chemical Bonds:
- Ionic Bonds: Formed when one atom donates electrons to another, resulting in charged ions. Example: NaCl (sodium chloride).
- Covalent Bonds: Formed when two atoms share electrons. Example: H₂O (water).
- Metallic Bonds: Occur between metal atoms, where electrons are shared in a “sea” of electrons.
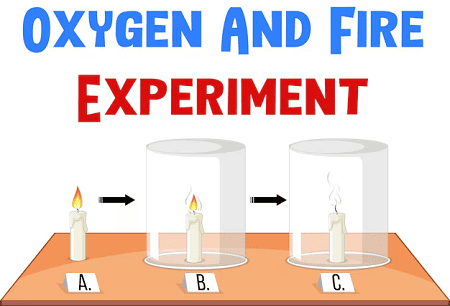
- Chemical Reactions: Processes in which reactants are transformed into products. This involves the breaking and forming of bonds. Chemical reactions are often represented by chemical equations, such as:2H2+O2→2H2O2H_2 + O_2 \rightarrow 2H_2O2H2+O2→2H2O(Hydrogen reacts with oxygen to form water.)
- Conservation of Mass: In a chemical reaction, matter is neither created nor destroyed, only rearranged.
- Moles and Molar Mass:
- Mole: A unit that represents a quantity of substance, equivalent to 6.022×10236.022 \times 10^{23}6.022×1023 particles (Avogadro’s number).
- Molar Mass: The mass of one mole of a substance, typically measured in grams per mole (g/mol).
- Atomic Number and Mass Number:
- Atomic Number: The number of protons in the nucleus of an atom, which defines the element.
- Mass Number: The sum of protons and neutrons in an atom’s nucleus.
- The Periodic Table: A tabular arrangement of elements, organized by increasing atomic number. Elements in the same column (group) have similar chemical properties.
- Acids and Bases:
- Acids: Substances that release hydrogen ions (H⁺) when dissolved in water. Example: HCl (hydrochloric acid).
- Bases: Substances that release hydroxide ions (OH⁻) in water. Example: NaOH (sodium hydroxide).
- The pH scale measures the acidity or basicity of a solution, with values ranging from 0 (acidic) to 14 (basic).
- Solutions: Homogeneous mixtures of two or more substances. The substance present in the greatest amount is the solvent, and the other substances are solutes.
These concepts provide a foundational understanding of chemistry and serve as the basis for exploring more advanced topics.
What is Some Basic Concepts of Chemistry ?
Some basic concepts of chemistry are essential for understanding how matter behaves, interacts, and changes. Here are key concepts:
- Matter: Anything that has mass and occupies space. It can exist in three states: solid, liquid, and gas.
- Atoms and Elements:
- Atoms: The smallest unit of matter that retains the properties of an element. Atoms are made up of protons, neutrons, and electrons.
- Elements: Pure substances made up of only one type of atom. Each element has unique properties and is listed in the Periodic Table (e.g., hydrogen, oxygen).
- Molecules and Compounds:
- Molecules: Two or more atoms chemically bonded together. Molecules can consist of the same element (e.g., O₂) or different elements (e.g., H₂O).
- Compounds: Substances made up of two or more different elements bonded together (e.g., water, NaCl).
- Chemical Bonds:
- Ionic Bonds: Formed when one atom donates an electron to another, creating positive and negative ions that attract each other (e.g., NaCl).
- Covalent Bonds: Occur when two atoms share electrons (e.g., H₂O).
- Metallic Bonds: Found between metal atoms, where electrons are free to move.
- Chemical Reactions: Processes in which substances (reactants) are transformed into new substances (products) by breaking and forming chemical bonds. This is represented by chemical equations (e.g., 2H₂ + O₂ → 2H₂O).
- Conservation of Mass: In any chemical reaction, the mass of the reactants is equal to the mass of the products.
- Moles and Molar Mass:
- Mole: A unit used to measure the amount of substance. One mole equals 6.022×10236.022 \times 10^{23}6.022×1023 particles (Avogadro’s number).
- Molar Mass: The mass of one mole of a substance, expressed in grams per mole (g/mol).
- Periodic Table: A chart that organizes all known elements by increasing atomic number and similar properties. Elements in the same group (column) have similar chemical behaviors.
- Acids and Bases:
- Acids: Substances that release hydrogen ions (H⁺) in water (e.g., HCl).
- Bases: Substances that release hydroxide ions (OH⁻) in water (e.g., NaOH).
- The pH scale measures the acidity or alkalinity of a solution, ranging from 0 (acidic) to 14 (basic).
- Solutions: Homogeneous mixtures where one substance (solute) is dissolved in another (solvent), such as salt dissolved in water.
These basic concepts form the foundation of chemistry and are critical for understanding chemical processes and reactions.
Who is required Some Basic Concepts of Chemistry ?
Anyone studying or working in fields related to science and technology is required to understand the basic concepts of chemistry. Some groups who particularly need this knowledge include:
- Students:
- Middle and High School Students: Those studying science or chemistry as part of their curriculum.
- College and University Students: Those pursuing degrees in chemistry, biology, environmental science, medicine, engineering, physics, and other sciences.
- Science Enthusiasts and Hobbyists: Individuals interested in learning about chemistry for personal knowledge or experimentation.
- Healthcare Professionals:
- Doctors, Nurses, and Pharmacists: Understanding basic chemistry is important for understanding how drugs work, patient treatments, and medical laboratory tests.
- Biochemists and Molecular Biologists: Those working in the field of health and medicine need an in-depth understanding of chemical processes in living organisms.
- Engineers:
- Chemical Engineers: They need to understand chemical reactions and processes to design efficient systems and solve engineering problems.
- Environmental Engineers: They study chemistry to address pollution, waste management, and resource management.
- Materials Engineers: They work with chemicals and materials to develop new substances and products.
- Researchers and Scientists: Individuals working in research, especially in fields like biochemistry, materials science, and pharmacology, rely on chemistry to develop new technologies, pharmaceuticals, and products.
- Manufacturing and Industrial Professionals:
- Chemists: Those involved in the production of chemicals, pharmaceuticals, cosmetics, and food products.
- Quality Control/Assurance Technicians: Understanding chemistry is crucial for ensuring the quality of products.
- Environmental Scientists:
- Understanding the chemical composition of the atmosphere, water, soil, and ecosystems is key to studying pollution, climate change, and environmental health.
- Consumers and General Public:
- A basic understanding of chemistry can help individuals make informed decisions in everyday life, such as choosing cleaning products, understanding nutritional information, or addressing health concerns.
In summary, anyone involved in scientific fields, industrial processes, healthcare, and daily life can benefit from understanding basic chemistry concepts.
When is required Some Basic Concepts of Chemistry ?
The basic concepts of chemistry are required at various stages and in different contexts, including:
- Early Education:
- Middle and High School: Chemistry is a foundational subject in secondary education. Students begin to learn the basic concepts of chemistry to understand the nature of matter, chemical reactions, and the physical world around them.
- Undergraduate Studies:
- College/University: Students pursuing degrees in chemistry, biology, medicine, environmental science, engineering, and related fields need to understand the fundamental chemistry concepts to progress in their specialized studies.
- Career and Professional Development:
- In Science and Research: Researchers and scientists in fields like biochemistry, pharmacology, materials science, and environmental science rely on basic chemistry concepts when conducting experiments, developing products, and analyzing data.
- In Healthcare: Healthcare professionals like doctors, nurses, and pharmacists need chemistry knowledge to understand biochemistry, pharmacology, drug interactions, and medical treatments.
- In Engineering: Chemical, environmental, and materials engineers need chemistry to design systems, address environmental issues, or create new products.
- In Manufacturing and Industry: Professionals in industries like pharmaceuticals, food, chemicals, and cosmetics must apply chemistry concepts to ensure product quality, safety, and efficacy.
- Real-World Applications:
- Daily Life: Chemistry knowledge is useful in everyday life for tasks such as cleaning, cooking, understanding medicine, and making safe decisions about food, health, and products.
- Environmental Awareness: Individuals may need chemistry concepts to understand topics like pollution, recycling, energy use, and sustainability in the context of environmental conservation.
- When Solving Problems:
- In Science or Engineering Problem-Solving: Whether it’s balancing a chemical equation, understanding a chemical reaction, or designing an industrial process, understanding chemistry is crucial for troubleshooting and resolving challenges.
- In Public Health and Safety: During outbreaks, natural disasters, or safety protocols, chemistry helps professionals understand the reactions of chemicals, environmental impacts, and how to safely manage hazardous materials.
- Career Transitions and Continuous Learning:
- For Professionals Seeking Specialization: Professionals like those in law, forensics, or environmental protection might need to understand chemistry to specialize in areas like patent law, chemical analysis, or environmental compliance.
- Lifelong Learning: Individuals involved in innovation, product development, or research need to revisit and apply basic chemistry concepts throughout their careers.
In summary, the basic concepts of chemistry are required at different points in education, professional careers, daily life, and whenever dealing with scientific, industrial, or health-related matters. Understanding chemistry is essential for making informed decisions, advancing knowledge, and solving complex problems.
Which is required Some Basic Concepts of Chemistry ?
The basic concepts of chemistry are required by various individuals and fields, depending on the context. Here’s a breakdown of who requires them and why:
1. Students
- Middle and High School Students: Basic chemistry concepts are part of the general science curriculum. These concepts provide the foundation for understanding how matter behaves and interacts.
- Undergraduate and Graduate Students: Students in fields like chemistry, biology, physics, environmental science, engineering, and health sciences need a solid understanding of chemistry to progress in their studies.
2. Healthcare Professionals
- Doctors, Nurses, Pharmacists: Basic chemistry helps healthcare professionals understand drug interactions, the biochemical processes in the human body, and how various medications work.
- Biochemists and Medical Researchers: They rely on chemistry concepts to understand the molecular mechanisms of disease, drug design, and diagnostic techniques.
3. Engineers
- Chemical Engineers: They apply chemistry to design processes that involve chemical reactions, materials processing, and product development.
- Environmental Engineers: Understanding chemistry is essential for dealing with pollution control, waste management, and resource management.
- Materials Engineers: They need to understand chemical properties to create new materials and improve existing ones.
4. Researchers and Scientists
- Chemists and Biochemists: Researchers in these fields use chemistry to explore new compounds, develop materials, and understand molecular interactions.
- Environmental Scientists: They need chemistry to study pollutants, ecosystems, and environmental processes like acid rain, climate change, and water purification.
5. Manufacturers and Industrial Professionals
- Pharmaceutical Manufacturers: Basic chemistry is needed to ensure the production of safe and effective drugs.
- Food and Beverage Industry: Chemistry concepts are crucial for understanding food preservation, flavor development, and nutritional value.
- Cosmetic Manufacturers: Chemists in this field use basic chemistry to formulate skincare and beauty products.
6. General Public and Consumers
- Consumers: Basic chemistry knowledge helps individuals make informed decisions about the products they use, from household cleaners to personal care products.
- Environmental Awareness: An understanding of chemistry can help the public understand issues like recycling, pollution, and climate change.
7. Government and Regulatory Agencies
- Regulatory Bodies: Agencies such as the FDA, EPA, and OSHA need chemistry knowledge to regulate the safety of products, control pollution, and enforce safety standards in various industries.
8. Educators and Trainers
- Teachers and Trainers: Educators need to understand and communicate basic chemistry concepts to students at various levels, from school to university.
9. Lawyers and Forensic Scientists
- Patent Lawyers: For those working in areas of chemical patents, a basic understanding of chemistry is crucial.
- Forensic Scientists: They apply chemistry to analyze substances in crime investigations, such as blood, drugs, and other materials.
10. Innovators and Entrepreneurs
- Inventors and Entrepreneurs: Those involved in product development, especially in tech, pharmaceuticals, or green energy, need to understand the chemical properties of materials and processes to innovate effectively.
In summary, anyone involved in science, medicine, engineering, environmental issues, manufacturing, or daily life decisions can benefit from a basic understanding of chemistry. It’s required for both learning and practical applications in various fields.

How is required Some Basic Concepts of Chemistry ?
The basic concepts of chemistry are required in a variety of ways, depending on the context and the individuals involved. Here’s how these concepts are typically needed:
1. For Learning and Education:
- Foundation for Advanced Studies: In schools, colleges, and universities, basic chemistry concepts form the foundation for more advanced studies in chemistry, biology, physics, and other sciences. Understanding core principles like atoms, molecules, chemical bonding, and reactions is essential for grasping higher-level topics.
- Critical Thinking and Problem-Solving: Chemistry helps students develop analytical and problem-solving skills. Concepts such as balancing chemical equations, calculating molar masses, and understanding reaction rates help students approach problems logically and methodically.
2. For Practical Applications in Science and Industry:
- Chemical Reactions: Knowledge of basic chemistry is required to understand and control chemical reactions in industries like pharmaceuticals, manufacturing, and food production. This is crucial for designing products, optimizing processes, and ensuring safety.
- Product Formulation: In industries such as cosmetics, cleaning products, and food, basic chemistry is needed to formulate new products or improve existing ones. This includes understanding the chemical properties of substances and how they interact.
- Quality Control and Assurance: Industries need chemistry concepts to ensure that products meet safety and quality standards. Basic chemistry is essential in testing and verifying the composition of raw materials and finished products.
3. For Health and Medicine:
- Understanding Biochemical Processes: Healthcare professionals, such as doctors, nurses, and pharmacists, require a knowledge of basic chemistry to understand the chemical processes in the human body, how drugs interact, and how treatments work. For example, understanding the role of enzymes in metabolism or the way acids and bases affect bodily functions is crucial.
- Drug Development: Pharmaceutical chemists need to understand chemical reactions, molecular structures, and pharmacodynamics to develop safe and effective drugs. Basic chemistry helps in drug design, formulation, and testing.
4. For Environmental Understanding:
- Pollution Control: Understanding the chemistry of pollutants, chemical cycles in nature, and environmental chemistry is crucial for addressing issues like air and water pollution, waste management, and climate change.
- Sustainability and Green Chemistry: As industries move towards more sustainable practices, knowledge of basic chemistry is required to design greener processes and products that minimize environmental harm.
5. For Everyday Life:
- Cooking and Food Safety: Chemistry concepts are needed in cooking (e.g., understanding how heat changes the structure of food) and in food safety (e.g., understanding preservatives and contaminants).
- Household and Cleaning: Understanding the chemistry behind cleaning products (like soaps, detergents, or disinfectants) and their reactions with different materials helps in making effective and safe choices.
- Personal Care: Chemistry helps in understanding how personal care products like skincare, hair care, and cosmetics work, and it aids in the formulation and proper use of these products.
6. For Innovation and Technological Advancements:
- New Materials and Technology: Basic chemistry is required for the development of new materials and technologies. From semiconductors to nanotechnology, understanding chemical processes is essential for innovation in these fields.
- Energy Solutions: Chemistry plays a vital role in developing new sources of energy, such as batteries, fuel cells, and renewable energy technologies. A knowledge of chemical reactions and material properties is key to improving energy storage and efficiency.
7. For Problem Solving and Troubleshooting:
- Understanding Reactions: When problems arise in chemical or industrial processes (such as unexpected reactions, product failure, or contamination), a basic understanding of chemistry helps in diagnosing the issue and finding solutions.
- Balancing Chemical Equations: In laboratory or industrial settings, balancing chemical equations is necessary for scaling reactions and ensuring the correct proportions of materials are used.
8. For Communication and Collaboration:
- Effective Communication in Science: Scientists, researchers, and professionals in any science-related field need basic chemistry knowledge to effectively communicate findings, share research, and collaborate on projects.
- Collaborating Across Disciplines: Chemistry is integral to fields like biology, physics, environmental science, and engineering. Professionals in these fields need a shared understanding of chemistry to collaborate and work on interdisciplinary projects.
9. For Regulation and Safety:
- Regulatory Compliance: Professionals in industries such as pharmaceuticals, food production, and chemicals need basic chemistry knowledge to meet regulatory requirements and ensure public safety.
- Hazardous Materials Management: Understanding the chemical properties of materials, such as flammability, toxicity, and reactivity, is essential for handling and disposing of hazardous substances safely.
10. For Personal Growth and Awareness:
- Curiosity and Lifelong Learning: Basic chemistry knowledge helps foster curiosity about how the world works. It allows individuals to ask informed questions and make well-reasoned decisions about everything from health to environmental issues.
- Informed Consumer Choices: Basic chemistry helps consumers make informed choices about the products they buy, from cleaning products to medicines, ensuring they understand the composition, usage, and safety of everyday items.
In Summary:
Basic concepts of chemistry are required in a wide range of contexts, from education and scientific research to everyday problem-solving and innovation. Whether it’s understanding chemical reactions, developing products, making health decisions, or contributing to environmental sustainability, chemistry plays a crucial role in improving knowledge and enabling practical solutions across various fields.
Case study is Some Basic Concepts of Chemistry ?
A case study in the context of “Some Basic Concepts of Chemistry” involves applying foundational chemistry concepts to real-world situations. Here’s an example of how basic chemistry principles are used to address practical challenges, focusing on a particular scenario:
Case Study: Water Purification and Chemical Treatment
Background:
A small community located near a river is facing water contamination due to industrial waste being dumped into the water. The water contains high levels of heavy metals like lead, arsenic, and mercury, as well as excess nutrients leading to algal blooms. The local government is working with a team of chemists and environmental engineers to develop a cost-effective and efficient water treatment solution.
Key Chemistry Concepts Involved:
- Chemical Reactions:
- The process of precipitation is used to remove dissolved metals from the water. By adding specific chemicals, such as sodium hydroxide (NaOH), the dissolved metals like lead (Pb²⁺) form insoluble compounds that can be easily filtered out. This is based on the principle of solubility and chemical reactions.
- Acid-Base Chemistry:
- The pH of water is a critical factor in many chemical processes. Acid-base reactions are used to adjust the pH level of the water. For example, lime (calcium hydroxide, Ca(OH)₂) is added to neutralize acidic water, making it safe for consumption and improving the effectiveness of coagulation and precipitation processes.
- Oxidation-Reduction (Redox) Reactions:
- To remove arsenic (As³⁺), an oxidation process is used. Arsenic ions in water can be oxidized using chemicals like ferric chloride (FeCl₃) to form arsenic(V), which can then be precipitated and filtered out. This process is essential because arsenic in its trivalent form is more toxic than in its pentavalent form.
- Flocculation and Coagulation:
- The addition of coagulants like aluminum sulfate (alum) helps in removing excess nutrients and suspended solids. Coagulation involves the addition of chemicals to the water that cause the tiny particles to stick together, forming flocs that can be removed through filtration or sedimentation. This helps in cleaning up the water, particularly when it is cloudy due to suspended particles.
- Chemical Disinfection:
- To ensure the water is free of pathogens, chlorine or ozone is added as a disinfectant. Chlorine works through oxidation, killing harmful microorganisms. It’s a fundamental application of redox chemistry to purify water and ensure it is safe for human consumption.
- Chemical Balance:
- Throughout the treatment process, it’s essential to maintain a chemical balance in the water, ensuring that harmful chemicals (like chlorine or aluminum) are present at safe levels. Monitoring and adjusting the concentrations of various chemicals helps achieve the desired water quality.
Implementation of Chemistry Concepts:
- The water treatment team conducts tests to measure the concentration of contaminants, pH levels, and the effectiveness of various chemicals.
- Using basic chemistry principles like solubility, acid-base reactions, and oxidation-reduction, the team adjusts chemical dosages to optimize the purification process.
- The team also monitors the effects of the treatment process on the environment, ensuring that the chemicals used do not harm the aquatic ecosystem after being released back into the environment.
Results:
After applying these basic chemistry principles:
- The water is treated effectively, with reduced levels of heavy metals, pathogens, and suspended solids.
- The community now has access to cleaner and safer drinking water.
- The process also minimizes environmental impact, as the treated water contains fewer toxic chemicals when released back into the river.
Conclusion:
This case study demonstrates how basic chemistry concepts like chemical reactions, acid-base chemistry, redox reactions, and coagulation can be applied to solve real-world environmental problems. Through the understanding of fundamental principles, chemists and engineers can devise effective solutions for water treatment and purification, ensuring public health and environmental safety.
In this case, Some Basic Concepts of Chemistry were directly applied to address a critical issue, showcasing the importance of chemistry in environmental science, public health, and engineering.
White paper on Some Basic Concepts of Chemistry ?
White Paper: Some Basic Concepts of Chemistry
Introduction
Chemistry is the branch of science that deals with the study of matter, its properties, its composition, and the reactions it undergoes. As one of the core natural sciences, chemistry plays a crucial role in various fields such as medicine, engineering, environmental science, agriculture, and many more. Understanding the fundamental concepts of chemistry provides a foundation for more advanced scientific learning and practical applications. This white paper aims to explore the essential basic concepts of chemistry and highlight their importance in both scientific education and real-world applications.
1. Atomic Structure and Chemical Bonding
The atom is the basic unit of matter. An atom consists of three primary subatomic particles: protons, neutrons, and electrons. The nucleus, composed of protons and neutrons, is at the center of the atom, while electrons orbit around the nucleus in shells or energy levels.
Key concepts:
- Atomic Number: The number of protons in an atom’s nucleus, which determines the element.
- Mass Number: The total number of protons and neutrons in an atom’s nucleus.
- Isotopes: Atoms of the same element that differ in the number of neutrons.
- Electron Configuration: The arrangement of electrons in an atom’s energy levels, determining its chemical properties.
Chemical Bonding: Atoms combine to form molecules through chemical bonds. The two main types of chemical bonds are:
- Covalent Bonding: Involves the sharing of electrons between atoms, as seen in molecules like water (H₂O).
- Ionic Bonding: Involves the transfer of electrons from one atom to another, leading to the formation of charged ions, as seen in sodium chloride (NaCl).
2. The Mole Concept and Molar Mass
The mole is a fundamental concept in chemistry that provides a bridge between the atomic and macroscopic worlds. It is used to count entities like atoms, molecules, or ions by relating them to a set number (Avogadro’s number, approximately 6.022×10236.022 \times 10^{23}6.022×1023).
- Molar Mass: The mass of one mole of a substance, usually expressed in grams per mole (g/mol). It is numerically equivalent to the atomic or molecular mass, but in grams.
This concept allows chemists to measure quantities of substances and calculate amounts needed for chemical reactions.
3. Chemical Reactions and Stoichiometry
A chemical reaction involves the transformation of reactants into products. The reactants are chemically altered to form new substances. A chemical equation represents a reaction and must obey the law of conservation of mass, meaning matter cannot be created or destroyed.
Key concepts:
- Balancing Chemical Equations: Ensuring that the number of atoms of each element is the same on both sides of the equation.
- Stoichiometry: The calculation of reactants and products in chemical reactions based on the relationships defined by a balanced chemical equation.
Through stoichiometry, chemists can determine how much of each reactant is needed to produce a given amount of product.
4. Thermodynamics and Energy in Chemical Reactions
Thermodynamics is the study of energy changes in chemical reactions. Energy is required to break bonds in reactants, and energy is released when new bonds are formed in products. Understanding the flow of energy in reactions is essential for controlling and optimizing chemical processes.
Key concepts:
- Exothermic Reactions: Reactions that release energy (usually in the form of heat).
- Endothermic Reactions: Reactions that absorb energy.
- Enthalpy (ΔH): A measure of the total heat content of a system.
- Entropy (ΔS): A measure of disorder or randomness in a system.
- Gibbs Free Energy (ΔG): A function that predicts the spontaneity of a reaction.
5. Acids, Bases, and pH Scale
Acids are substances that release hydrogen ions (H⁺) when dissolved in water, while bases release hydroxide ions (OH⁻). The pH scale is used to measure the acidity or basicity of a solution, ranging from 0 (acidic) to 14 (basic), with 7 being neutral.
Key concepts:
- Bronsted-Lowry Theory: Acids are proton donors, and bases are proton acceptors.
- Arrhenius Theory: Acids produce H⁺ ions in water, and bases produce OH⁻ ions.
- Buffer Solutions: Solutions that resist changes in pH when small amounts of acid or base are added, maintaining a stable environment.
Understanding acids, bases, and pH is essential for processes such as controlling the acidity of rainwater, industrial processes, and biological systems.
6. Chemical Kinetics and Reaction Rates
Chemical kinetics is the study of the rates at which chemical reactions occur and the factors that influence them. The rate of a reaction depends on several factors, including temperature, concentration, surface area, and the presence of a catalyst.
Key concepts:
- Rate of Reaction: The change in concentration of reactants or products per unit of time.
- Activation Energy: The minimum energy required for a reaction to occur.
- Catalysts: Substances that speed up the rate of a chemical reaction without being consumed in the process.
7. Chemical Equilibrium
In a reversible chemical reaction, both the forward and reverse reactions occur at the same time, and the system reaches a state where the concentrations of reactants and products remain constant. This is known as chemical equilibrium.
Key concepts:
- Le Chatelier’s Principle: States that if a system at equilibrium is disturbed, it will shift in the direction that counteracts the disturbance.
- Equilibrium Constant (K): A numerical value that represents the ratio of the concentrations of products to reactants at equilibrium.
8. Organic Chemistry
Organic chemistry deals with the study of carbon-containing compounds, which form the basis for life. These compounds can be natural (like proteins, carbohydrates, and lipids) or synthetic (like plastics, drugs, and dyes).
Key concepts:
- Hydrocarbons: Compounds consisting solely of hydrogen and carbon atoms.
- Functional Groups: Specific groups of atoms that give organic compounds their characteristic properties (e.g., alcohols, acids, amines).
- Isomerism: The occurrence of compounds with the same molecular formula but different structural arrangements.
Conclusion
Basic chemistry concepts are essential not only for scientific study but also for their practical applications in everyday life, industry, medicine, and environmental science. From understanding atomic structure to mastering the principles of chemical reactions, these concepts lay the foundation for more complex studies and innovations that drive modern society. As we continue to face global challenges in health, energy, and sustainability, a solid understanding of the fundamental concepts of chemistry will be critical in finding solutions.
References
- Brown, T.L., LeMay, H.E., Bursten, B.E., & Chang, D. (2009). Chemistry: The Central Science.
- Zumdahl, S.S., & Zumdahl, S.A. (2017). Chemistry: An Atoms First Approach.
- Atkins, P., & de Paula, J. (2014). Physical Chemistry (10th ed.).
This white paper outlines the basic chemistry concepts that are essential for scientific development and practical applications in various industries and environmental challenges.
Industrial application of Some Basic Concepts of Chemistry ?
Industrial Application of Some Basic Concepts of Chemistry
Chemistry is foundational to numerous industrial processes, driving innovation, improving efficiency, and ensuring safety in the production of various goods and services. The basic concepts of chemistry such as atomic structure, chemical reactions, thermodynamics, acids and bases, and chemical bonding are applied in industries ranging from pharmaceuticals to manufacturing, agriculture, energy production, and environmental management. This section outlines several key industrial applications of basic chemistry concepts.
1. Pharmaceutical Industry: Drug Development and Production
The pharmaceutical industry relies heavily on chemistry to design, synthesize, and produce drugs. The basic concepts of chemical reactions, molecular structure, and chemical bonding are essential in understanding how drugs interact with the body and how to optimize their effectiveness.
- Chemical Reactions: Pharmaceutical companies use controlled chemical reactions to synthesize active ingredients. For example, reactions like esterification, oxidation-reduction, and polymerization are common in the creation of drugs.
- Molecular Structure and Bonding: Understanding molecular structure and bonding helps in designing drugs that can specifically target and interact with biological molecules, such as enzymes or receptors.
- Pharmacokinetics: The study of how drugs are absorbed, distributed, metabolized, and excreted in the body is based on principles of thermodynamics, including the concept of activation energy and reaction rates.
Example: The development of antibiotics like penicillin involves synthesizing complex organic molecules through chemical reactions, leveraging knowledge of molecular bonding and functional groups.
2. Agriculture: Fertilizers, Pesticides, and Herbicides
The agriculture industry makes extensive use of chemistry, particularly in the development of fertilizers, pesticides, and herbicides, to enhance crop production and protect plants from pests.
- Acid-Base Chemistry: Fertilizers such as ammonium nitrate (NH₄NO₃) rely on principles of acid-base chemistry. The balance of pH in soils is essential for nutrient absorption by plants, so understanding how different acids and bases interact with the environment is crucial.
- Chemical Reactions and Synthesis: The production of herbicides and pesticides involves chemical reactions that target the metabolic processes of pests or weeds. For example, organophosphate pesticides affect the nervous system of insects through phosphorylation, a chemical reaction.
- Molecular Interactions: Chemistry is used to design molecules that selectively target specific pests or plants without harming others, optimizing the effectiveness of agricultural chemicals while minimizing environmental impact.
Example: NPK fertilizers, which provide essential nutrients like nitrogen (N), phosphorus (P), and potassium (K), are chemically synthesized through reactions such as the Haber-Bosch process for nitrogen fixation.
3. Energy and Petrochemical Industry: Fuels, Energy Production, and Chemical Engineering
The energy industry is deeply intertwined with basic chemistry principles, particularly in the production of fuels, renewable energy, and the chemical processes used in refining oil and natural gas.
- Chemical Reactions and Combustion: Combustion reactions, which are exothermic reactions between fuel and oxygen, are fundamental in energy production. For instance, burning fossil fuels like coal, oil, and natural gas releases energy that is used to produce electricity and heat.
- Thermodynamics: The principles of thermodynamics, such as enthalpy, entropy, and Gibbs free energy, are used to understand and optimize energy production processes, such as refining oil or generating electricity in power plants.
- Catalysis: In the production of fuels like gasoline, catalysts are used in processes like cracking and alkylation to break down large hydrocarbons into smaller, more useful molecules.
Example: The Haber-Bosch process not only plays a role in fertilizer production but also contributes to the production of ammonia for nitric acid and urea, which are important for various energy-related applications.
4. Environmental Chemistry: Pollution Control and Waste Management
Chemistry plays a critical role in environmental protection, from waste treatment to pollution control, as industries work to reduce their ecological footprints and comply with environmental regulations.
- Acid-Base Reactions: Neutralization reactions are commonly used in water treatment processes, where acidic or alkaline water is treated with basic or acidic chemicals to reach a neutral pH, making the water safer for consumption or discharge.
- Redox Reactions: In waste treatment plants, oxidation-reduction (redox) reactions are used to break down toxic compounds, such as in the treatment of sewage or industrial wastewater. For example, ozone treatment is used to purify water by oxidizing contaminants.
- Catalysis: The use of catalysts in industrial processes reduces harmful emissions. Catalytic converters in automobiles use platinum, palladium, and rhodium to convert toxic gases like carbon monoxide (CO) into harmless carbon dioxide (CO₂) and water.
Example: In the scrubbing of sulfur dioxide (SO₂) from power plant emissions, lime (CaO) is used to neutralize sulfuric acid and remove harmful compounds from exhaust gases.
5. Manufacturing: Polymers and Materials Science
The manufacturing industry uses chemistry to create polymers and materials with specific properties for a wide range of applications, from packaging to electronics.
- Polymerization: The process of making polymers from monomers involves chemical reactions like addition polymerization or condensation polymerization. The properties of the resulting polymer, such as flexibility or strength, depend on the chemical structure and bonding of the monomers.
- Chemical Bonding: Understanding chemical bonding allows for the creation of new materials with specific properties. For instance, epoxy resins are used in adhesives, coatings, and composite materials, relying on knowledge of covalent bonds and cross-linking.
- Catalysts: Catalysts are employed in the production of plastics and synthetic fibers, optimizing the polymerization processes and reducing the environmental impact.
Example: The production of polyethylene, used in everything from plastic bags to containers, involves the polymerization of ethylene (C₂H₄) through a free-radical process, relying on basic concepts of chemical bonding and reaction mechanisms.
6. Food and Beverage Industry: Preservation, Flavoring, and Additives
In the food industry, chemistry is used to preserve food, enhance flavors, and improve the nutritional value of products. Various basic chemistry concepts are applied in food preservation, fermentation, and flavor enhancement.
- Acid-Base Chemistry: The pickling process, which involves preserving food in acidic solutions, relies on the principles of acid-base chemistry. The acidity in pickled foods helps prevent the growth of bacteria.
- Fermentation: Fermentation, a biochemical process involving microbial metabolism, is used in the production of alcoholic beverages, yogurt, and bread. It is a chemical process that produces energy in the absence of oxygen, releasing compounds like ethanol and lactic acid.
- Emulsification: The formation of stable mixtures of oil and water (such as in mayonnaise) relies on the concept of emulsification, where surfactants are used to stabilize the mixture.
Example: The process of canning uses heat to destroy microorganisms in food, preserving it for long periods. The chemistry of heat transfer and the Maillard reaction (responsible for browning and flavor development) are fundamental in this process.
Conclusion
The basic concepts of chemistry are indispensable in driving innovation and improving efficiency across various industries. From pharmaceutical development and agricultural practices to energy production and environmental protection, chemistry provides the tools and insights needed to solve complex challenges. By applying principles such as chemical reactions, thermodynamics, acid-base chemistry, and catalysis, industries can develop new products, improve existing processes, and ensure sustainability in their operations.
Understanding these concepts not only supports scientific progress but also facilitates the creation of solutions that impact the economy, society, and the environment on a global scale.
Research and development of Some Basic Concepts of Chemistry ?
Research and Development of Some Basic Concepts of Chemistry
Research and development (R&D) in the field of chemistry plays a crucial role in advancing scientific knowledge, improving existing technologies, and fostering innovation across industries. The development of basic chemistry concepts, such as atomic structure, chemical bonding, thermodynamics, chemical reactions, and kinetics, forms the foundation for a wide range of innovations, from new materials to medical treatments and environmental solutions. Below are the key areas where R&D in basic concepts of chemistry is actively shaping the future.
1. Advanced Materials and Nanotechnology
The development of new materials is one of the most active areas of research in chemistry, often relying on deep insights into atomic structure, chemical bonding, and crystal engineering. The goal is to create materials with unique properties that can be used in electronics, medicine, energy storage, and other applications.
- Nanomaterials: Research in nanotechnology focuses on the synthesis and application of materials at the nanoscale (typically between 1 and 100 nanometers). The properties of materials change significantly at the nanoscale due to quantum effects and surface area to volume ratio. For example, carbon nanotubes exhibit exceptional strength and conductivity due to their unique bonding structure.
- Polymer Chemistry: Advances in polymer science are driven by understanding polymerization mechanisms (addition and condensation polymerization) and how chemical structure influences the physical properties of polymers. Research in this area is leading to smart polymers that respond to environmental stimuli (e.g., pH, temperature).
Example: Researchers are developing graphene, a single layer of carbon atoms arranged in a hexagonal lattice, which is extremely strong and highly conductive, making it ideal for applications in electronics, energy storage, and medical devices.
2. Green Chemistry and Sustainable Development
One of the major goals of contemporary chemistry R&D is to create processes and products that are environmentally sustainable. Green chemistry focuses on designing chemical processes that reduce waste, energy consumption, and the use of hazardous substances.
- Renewable Energy: R&D in solar cells, batteries, and biofuels depends on an understanding of chemical reactions and thermodynamics to improve the efficiency of energy storage and energy conversion.
- Catalysis: Green chemistry aims to develop catalysts that enable reactions to occur under milder conditions, reducing the need for harsh chemicals and high temperatures. Enzymatic catalysis, inspired by natural biological processes, is a growing area of research.
- Waste Management: Researchers are developing ways to recycle or repurpose waste materials using chemical reactions that transform pollutants into useful byproducts. This includes efforts to recycle plastics and reduce environmental impact from industrial processes.
Example: The development of catalytic converters in automobiles is a direct application of green chemistry, as it allows for the reduction of harmful emissions through catalytic reactions, converting toxic gases into less harmful substances.
3. Pharmaceuticals and Drug Development
Pharmaceutical R&D is another area where basic chemistry concepts are crucial. Chemical reactions, molecular structure, and chemical bonding are central to understanding how drugs interact with biological systems.
- Drug Design and Synthesis: Research in medicinal chemistry involves designing molecules that can interact with specific biological targets (e.g., enzymes, receptors) to treat diseases. This often requires understanding of stereochemistry (the 3D arrangement of atoms), functional groups, and reaction mechanisms.
- Biotechnology and Biochemistry: R&D in biotechnology often combines chemistry with biology, such as developing drugs through biochemical synthesis or using biochemical pathways for producing pharmaceuticals.
- Pharmacokinetics and Drug Delivery: Researchers use basic concepts of chemistry, including solubility, diffusion, and molecular interactions, to develop effective drug delivery systems, ensuring that drugs reach their intended target at the correct dose.
Example: The development of targeted therapies for cancer, such as monoclonal antibodies and small molecule inhibitors, relies on understanding how drugs can selectively bind to cancer cells without affecting healthy cells, based on chemical bonding and molecular interactions.
4. Environmental Chemistry and Climate Change Solutions
Environmental chemistry is a field focused on understanding and mitigating the impact of human activities on the environment. Research here involves applying fundamental chemistry principles to tackle challenges like pollution, climate change, and resource depletion.
- Atmospheric Chemistry: R&D into the chemical processes that occur in the atmosphere, such as the breakdown of pollutants (e.g., ozone-depleting chemicals) and the interaction of greenhouse gases with solar radiation, is critical for understanding climate change.
- Carbon Capture and Storage (CCS): Researchers are working on chemical methods to capture carbon dioxide (CO₂) emissions from industrial processes and store them safely, preventing their release into the atmosphere.
- Water Purification: Chemists are developing new materials and chemical processes to purify water. For example, adsorption techniques and advanced oxidation processes rely on a deep understanding of chemical reactions to remove contaminants from water.
Example: The development of photocatalytic materials to break down pollutants in water using light is an emerging area of research. These materials rely on principles of semiconductor chemistry and photo-induced electron transfer.
5. Chemical Engineering and Process Optimization
Chemical engineering research focuses on improving the efficiency, safety, and scalability of chemical processes. Basic chemistry concepts such as reaction kinetics, thermodynamics, and equilibrium are crucial for optimizing industrial processes.
- Reaction Kinetics: Understanding the rates at which chemical reactions occur helps in optimizing industrial processes, whether it’s in the production of chemicals, pharmaceuticals, or materials. Research into reaction pathways and activation energy allows for more efficient use of energy and raw materials.
- Process Engineering: R&D in process engineering focuses on scaling up laboratory reactions to industrial levels while maintaining efficiency and minimizing waste. Heat transfer, mass transfer, and fluid dynamics are central to this research.
- Separation Technologies: Researchers are constantly developing new methods for separating and purifying chemical products. This includes processes such as distillation, membrane filtration, and chromatography, which are vital in industries like petrochemicals, food processing, and pharmaceuticals.
Example: Polymerization reactors used in the manufacturing of plastics must be optimized for reaction rates and heat removal to ensure high product yields and energy efficiency. Research in catalytic reactors improves the production process by controlling reaction conditions and minimizing byproducts.
6. Analytical Chemistry and Instrumentation
Analytical chemistry involves developing and applying techniques to detect and quantify substances in various materials. Research in this area often involves the development of new instrumentation and techniques for precise analysis.
- Spectroscopy: Research into spectroscopic techniques such as NMR (nuclear magnetic resonance), mass spectrometry, and IR (infrared) spectroscopy has led to highly accurate methods for identifying the molecular structure of compounds.
- Chromatography: Advancements in chromatography techniques, including gas chromatography (GC) and high-performance liquid chromatography (HPLC), are important for separating and analyzing complex mixtures.
- Sensors and Diagnostics: Development of biosensors and chemical sensors allows for the detection of specific molecules, often at low concentrations, in environmental monitoring, medical diagnostics, and food safety.
Example: In environmental monitoring, portable sensors based on chemical reactions are used to detect pollutants like heavy metals or volatile organic compounds in air or water, helping to enforce environmental regulations.
Conclusion
Research and development in the field of basic chemistry concepts is driving innovations across multiple industries, from pharmaceuticals and materials science to environmental sustainability and energy production. Fundamental concepts like atomic structure, chemical reactions, thermodynamics, and catalysis serve as the foundation for developing new technologies that enhance our daily lives and address global challenges.
The future of R&D in chemistry promises further breakthroughs that will improve the efficiency of industrial processes, create novel materials, advance healthcare, and provide solutions to environmental issues, fostering a more sustainable and technologically advanced world.
Courtesy : Physics Wallah – Alakh Pandey
References
^ Brown, Theodore L.; LeMay, H. Eugene Jr.; Bursten, Bruce E.; Murphey, Catherine J.; Woodward, Patrick M.; Stoltzfus, Matthew W.; Lufaso, Michael W. (2018). “Introduction: Matter, energy, and measurement”. Chemistry: The Central Science (14th ed.). New York: Pearson. pp. 46–85. ISBN 978-0134414232.
^ “What is Chemistry?”. Chemweb.ucc.ie. Archived from the original on 3 October 2018. Retrieved 12 June 2011.
^ “Definition of CHEMISTRY”. Merriam-Webster. Archived from the original on 7 August 2020. Retrieved 24 August 2020.
^ “Definition of chemistry | Dictionary.com”. www.dictionary.com. Archived from the original on 5 March 2016. Retrieved 24 August 2020.
^ “Chemistry Is Everywhere”. American Chemical Society. Archived from the original on 29 November 2020. Retrieved 1 December 2020.
^ Carsten Reinhardt. Chemical Sciences in the 20th Century: Bridging Boundaries. Wiley-VCH, 2001. ISBN 3-527-30271-9. pp. 1–2.
^ Theodore L. Brown, H. Eugene Lemay, Bruce Edward Bursten, H. Lemay. Chemistry: The Central Science. Prentice Hall; 8 ed. (1999). ISBN 0-13-010310-1. pp. 3–4.
^ “Chemistry – Chemistry and society”. Britannica. Archived from the original on 6 May 2023. Retrieved 6 May 2023.
^ Ihde, Aaron J. (1984). The development of modern chemistry. New York: Dover. ISBN 978-0-486-64235-2.
^ Newman, William R. (2011). “What Have We Learned from the Recent Historiography of Alchemy?”. Isis. 102 (2): 313–321. doi:10.1086/660140. ISSN 0021-1753. PMID 21874691.
^ “alchemy”, entry in The Oxford English Dictionary, J.A. Simpson and E.S.C. Weiner, vol. 1, 2nd ed., 1989, ISBN 0-19-861213-3.
^ Weekley, Ernest (1967). Etymological Dictionary of Modern English. New York: Dover Publications. ISBN 0-486-21873-2.
^ “chemical bonding”. Britannica. Encyclopædia Britannica. Archived from the original on 26 April 2012. Retrieved 1 November 2012.
^ Anthony Carpi. Matter: Atoms from Democritus to Dalton, Archived 28 February 2007 at the Wayback Machine.
^ IUPAC, Gold Book Definition, Archived 4 March 2007 at the Wayback Machine.
^ “California Occupational Guide Number 22: Chemists”. Calmis.ca.gov. 29 October 1999. Archived from the original on 10 June 2011. Retrieved 12 June 2011.
^ “General Chemistry Online – Companion Notes: Matter”. Antoine.frostburg.edu. Archived from the original on 24 June 2011. Retrieved 12 June 2011.
^ Armstrong, James (2012). General, Organic, and Biochemistry: An Applied Approach. Brooks/Cole. p. 48. ISBN 978-0-534-49349-3.
^ Burrows et al. 2009, p. 13.
^ Jump up to:a b Housecroft & Sharpe 2008, p. 2.
^ Burrows et al. 2009, p. 110.
^ Burrows et al. 2009, p. 12.
^ “IUPAC Nomenclature of Organic Chemistry”. Acdlabs.com. Archived from the original on 8 June 2011. Retrieved 12 June 2011.
^ Connelly, Neil G.; Damhus, Ture; Hartshom, Richard M.; Hutton, Alan T. (2005). Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005. Cambridge: Royal Society of Chemistry Publishing / IUPAC. ISBN 0854044388. Retrieved 13 June 2022.
^ Hill, J. W.; Petrucci, R. H.; McCreary, T. W.; Perry, S. S. (2005). General Chemistry (4th ed.). Upper Saddle River, New Jersey: Pearson Prentice Hall. p. 37.
^ Avedesian, M. M.; Baker, Hugh. Magnesium and Magnesium Alloys. ASM International. p. 59.
^ Burrows et al. 2009, p. 16.
^ Atkins & de Paula 2009, p. 9.
^ “Chemical Bonding by Anthony Carpi, PhD”. visionlearning. Archived from the original on 17 July 2011. Retrieved 12 June 2011.
^ Reilly, Michael. (2007). Mechanical force induces chemical reaction, Archived 14 August 2014 at the Wayback Machine, NewScientist.com news service.
^ Changing States of Matter, Archived 28 April 2007 at the Wayback Machine, Chemforkids.com.
^ Chemical Reaction Equation, Archived 12 October 2007 at the Wayback Machine, IUPAC Goldbook.
^ Gold Book Chemical Reaction, Archived 4 March 2007 at the Wayback Machine, IUPAC Goldbook.
^ “History of Acidity”. BBC. 27 May 2004. Archived from the original on 27 February 2009. Retrieved 12 June 2011.
^ Jump up to:a b Principe, L. (2011). “In retrospect: The Sceptical Chymist”. Nature. 469 (7328): 30–31. Bibcode:2011Natur.469…30P. doi:10.1038/469030a. ISSN 1476-4687. S2CID 6490305.
^ “Selected Classic Papers from the History of Chemistry”. Archived from the original on 17 September 2018. Retrieved 8 October 2017.
^ Boyle, Robert (1661). The Sceptical Chymist. New York: Dover Publications, Incorporated (reprint). ISBN 978-0-486-42825-3.
^ Glaser, Christopher (1663). Traite de la chymie. Paris. as found in: Kim, Mi Gyung (2003). Affinity, That Elusive Dream – A Genealogy of the Chemical Revolution. The MIT Press. ISBN 978-0-262-11273-4.
^ Stahl, George (1730). Philosophical Principles of Universal Chemistry. London, England.
^ Dumas, J. B. (1837). ‘Affinite’ (lecture notes), vii, p. 4. “Statique chimique”, Paris, France: Académie des Sciences.
^ Pauling, Linus (1947). General Chemistry. Dover Publications, Inc. ISBN 978-0-486-65622-9.
^ Chang, Raymond (1998). Chemistry, 6th Ed. New York: McGraw Hill. ISBN 978-0-07-115221-1.
^ First chemists, Archived 8 January 2015 at the Wayback Machine, February 13, 1999, New Scientist.
^ Barnes, Ruth (2004). Textiles in Indian Ocean Societies. Routledge. p. 1. ISBN 978-0415297660.
^ Lucretius. “de Rerum Natura (On the Nature of Things)”. The Internet Classics Archive. Massachusetts Institute of Technology. Archived from the original on 29 June 2011. Retrieved 9 January 2007.
^ Simpson, David (29 June 2005). “Lucretius (c. 99–55 BCE)”. The Internet History of Philosophy. Archived from the original on 28 May 2010. Retrieved 10 November 2020.
^ Strodach, George K. (2012). The Art of Happiness. New York: Penguin Classics. pp. 7–8. ISBN 978-0-14-310721-7.
^ Fr. 12; see pp. 291–292 of Kirk, G. S.; Raven, J. E.; Schofield, Malcolm (1983). The Presocratic Philosophers (2nd ed.). Cambridge: Cambridge University Press. ISBN 978-0-521-27455-5.
^ Long, A. A.; Sedley, D. N. (1987). “Epicureanism: The principals of conservation”. The Hellenistic Philosophers. Vol 1: Translations of the principal sources with philosophical commentary. Cambridge: Cambridge University Press. pp. 25–26. ISBN 978-0-521-27556-9.
^ “International Year of Chemistry – The History of Chemistry”. G.I.T. Laboratory Journal Europe. 25 February 2011. Archived from the original on 15 June 2013. Retrieved 12 March 2013.
^ Bunch, Bryan H. & Hellemans, Alexander (2004). The History of Science and Technology. Houghton Mifflin Harcourt. p. 88. ISBN 978-0-618-22123-3.
^ Morris Kline (1985) Mathematics for the nonmathematician. Archived 5 September 2015 at the Wayback Machine. Courier Dover Publications. p. 284. ISBN 0-486-24823-2.
^ Marcelin Berthelot, Collection des anciens alchimistes grecs (3 vol., Paris, France, 1887–1888, p. 161); F. Sherwood Taylor, “The Origins of Greek Alchemy”, Ambix 1 (1937), p. 40.
^ Stapleton, Henry Enest; Azo, R. F.; Hidayat Husain, M. (1927). “Chemistry in Iraq and Persia in the Tenth Century A.D.” Memoirs of the Asiatic Society of Bengal. VIII (6): 317–418. OCLC 706947607. pp. 338–340; Kraus, Paul (1942–1943). Jâbir ibn Hayyân: Contribution à l’histoire des idées scientifiques dans l’Islam. I. Le corpus des écrits jâbiriens. II. Jâbir et la science grecque. Cairo: Institut Français d’Archéologie Orientale. ISBN 978-3-487-09115-0. OCLC 468740510. vol. II, pp. 41–42.
^ Darmstaedter, Ernst. “Liber Misericordiae Geber: Eine lateinische Übersetzung des gröβeren Kitâb l-raḥma”, Archiv für Geschichte der Medizin, 17/4, 1925, pp. 181–197; Berthelot, Marcellin. “Archéologie et Histoire des sciences”, Mémoires de l’Académie des sciences de l’Institut de France, 49, 1906, pp. 308–363; see also Forster, Regula. “Jābir b. Ḥayyān”, Archived 18 April 2021 at the Wayback Machine, Encyclopaedia of Islam, Three.
^ Newman, William R. “New Light on the Identity of Geber”, Sudhoffs Archiv, 1985, 69, pp. 76–90; Newman, William R. The Summa perfectionis of Pseudo-Geber: A critical ed., translation and study, Leiden: Brill, 1991, pp. 57–103. It has been argued by Ahmad Y. Al-Hassan that the pseudo-Geber works were actually translated into Latin from the Arabic (see Al-Hassan, Ahmad Y. “The Arabic Origin of the Summa and Geber Latin Works: A Refutation of Berthelot, Ruska, and Newman Based on Arabic Sources”, in: Ahmad Y. Al-Hassan. Studies in al-Kimya’: Critical Issues in Latin and Arabic Alchemy and Chemistry. Hildesheim: Georg Olms Verlag, 2009, pp. 53–104; also available online. Archived 25 February 2021 at the Wayback Machine).
^ Marmura, Michael E.; Nasr, Seyyed Hossein (1965). “An Introduction to Islamic Cosmological Doctrines. Conceptions of Nature and Methods Used for Its Study by the Ikhwan Al-Safa’an, Al-Biruni, and Ibn Sina by Seyyed Hossein Nasr”. Speculum. 40 (4): 744–746. doi:10.2307/2851429. JSTOR 2851429.
^ Robert Briffault (1938). The Making of Humanity, pp. 196–197.
^ Marshall, James L.; Marshall, Virginia R. (Autumn 2005). “Rediscovery of the Elements: Agricola” (PDF). The Hexagon. 96 (3). Alpha Chi Sigma: 59. ISSN 0164-6109. OCLC 4478114. Retrieved 7 January 2024.
^ Jump up to:a b “Georgius Agricola”. University of California – Museum of Paleontology. Retrieved 4 April 2019.
^ Jump up to:a b Rafferty, John P. (2012). Geological Sciences; Geology: Landforms, Minerals, and Rocks. New York: Britannica Educational Publishing, p. 10. ISBN 9781615305445
^ Karl Alfred von Zittel (1901). History of Geology and Palaeontology, p. 15.
^ “History – Robert Boyle (1627–1691)”. BBC. Archived from the original on 9 January 2011. Retrieved 12 June 2011.
^ Eagle, Cassandra T.; Sloan, Jennifer (1998). “Marie Anne Paulze Lavoisier: The Mother of Modern Chemistry”. The Chemical Educator. 3 (5): 1–18. doi:10.1007/s00897980249a. S2CID 97557390.
^ Kim, Mi Gyung (2003). Affinity, that Elusive Dream: A Genealogy of the Chemical Revolution. MIT Press. p. 440. ISBN 978-0-262-11273-4.
^ Davy, Humphry (1808). “On some new Phenomena of Chemical Changes produced by Electricity, particularly the Decomposition of the fixed Alkalies, and the Exhibition of the new Substances, which constitute their Bases”. Philosophical Transactions of the Royal Society. 98: 1–45. doi:10.1098/rstl.1808.0001. Archived from the original on 18 April 2021. Retrieved 30 November 2020.
^ “A Brief History of the Development of Periodic Table”. Chemistry 412 course notes. Western Oregon University. Archived from the original on 9 February 2020. Retrieved 20 July 2015.
^ Note. Archived 24 September 2015 at the Wayback Machine. “…it is surely true that had Mendeleev never lived modern chemists would be using a Periodic Table” and “Dmitri Mendeleev”. Royal Society of Chemistry. Archived from the original on 2 July 2014. Retrieved 18 July 2015.
^ Winter, Mark. “WebElements: the periodic table on the web”. The University of Sheffield. Archived from the original on 4 January 2014. Retrieved 27 January 2014.
^ “Julius Lothar Meyer and Dmitri Ivanovich Mendeleev”. Science History Institute. June 2016. Archived from the original on 21 March 2018. Retrieved 20 March 2018.
^ “What makes these family likenesses among the elements? In the 1860s everyone was scratching their heads about that, and several scientists moved towards rather similar answers. The man who solved the problem most triumphantly was a young Russian called Dmitri Ivanovich Mendeleev, who visited the salt mine at Wieliczka in 1859.” Bronowski, Jacob (1973). The Ascent of Man. Little, Brown and Company. p. 322. ISBN 978-0-316-10930-7.
^ Ihde, Aaron John (1984). The Development of Modern Chemistry. Courier Dover Publications. p. 164. ISBN 978-0-486-64235-2.
^ “Chemistry”. Chemistry2011.org. Archived from the original on 8 October 2011. Retrieved 10 March 2012.
^ “Chemistry Subdisciplines”. www.thecanadianencyclopedia.ca. Retrieved 1 April 2024.
^ “Analytical Chemistry”. American Chemical Society. Retrieved 1 April 2024.
^ Skoog, Douglas A.; Holler, F. James; Crouch, Stanley R. (2018). Principles of instrumental analysis (7th ed.). Australia: Cengage Learning. p. 120. ISBN 978-1-305-57721-3.
^ “Studying Biochemistry”. www.biochemistry.org. Retrieved 11 April 2024.
^ “Inorganic Chemistry”. American Chemical Society. Retrieved 1 April 2024.
^ Kaminsky, Walter (1 January 1998). “Highly active metallocene catalysts for olefin polymerization”. Journal of the Chemical Society, Dalton Transactions (9): 1413–1418. doi:10.1039/A800056E. ISSN 1364-5447.
^ “Polypropylene”. pslc.ws. Retrieved 11 April 2024.
^ Fahlman, Bradley D. (2011). Materials Chemistry (1st ed.). Dordrecht: Springer Netherlands Springer e-books Imprint: Springer. pp. 1–4. ISBN 978-94-007-0693-4.
^ “Nuclear Chemistry”. American Chemical Society. Retrieved 11 April 2024.
^ “21: Nuclear Chemistry”. Libretexts. 18 November 2014. Retrieved 11 April 2024.
^ “Little Boy and Fat Man – Nuclear Museum”. ahf.nuclearmuseum.org/. Retrieved 11 April 2024.
^ Brown, William Henry; Iverson, Brent L.; Anslyn, Eric V.; Foote, Christopher S. (2018). Organic chemistry (8th ed.). Boston, Massachusetts: Cengage Learning. p. 19. ISBN 978-1-305-58035-0.
^ Tullo, Alexander H. (28 July 2014). “C&EN’s Global Top 50 Chemical Firms For 2014”. Chemical & Engineering News. American Chemical Society. Archived from the original on 26 August 2014. Retrieved 22 August 2014. Helge Kragh (2000). Conceptual Changes in Chemistry: The Notion of a Chemical Element, ca. 1900-1925
^ Chemistry (IUPAC), The International Union of Pure and Applied. “IUPAC – chemical element (C01022)”. goldbook.iupac.org. doi:10.1351/goldbook.C01022.
^ See the timeline on p.10 in Oganessian, Yu. Ts.; Utyonkov, V.; Lobanov, Yu.; Abdullin, F.; Polyakov, A.; Sagaidak, R.; Shirokovsky, I.; Tsyganov, Yu. (2006). “Evidence for Dark Matter” (PDF). Physical Review C. 74 (4): 044602. Bibcode:2006PhRvC..74d4602O. doi:10.1103/PhysRevC.74.044602. Archived (PDF) from the original on 13 February 2021. Retrieved 8 October 2007.
^ “The Universe Adventure Hydrogen and Helium”. Lawrence Berkeley National Laboratory U.S. Department of Energy. 2005. Archived from the original on 21 September 2013.
^ astro.soton.ac.uk (3 January 2001). “Formation of the light elements”. University of Southampton. Archived from the original on 21 September 2013.
^ “How Stars Make Energy and New Elements” (PDF). Foothill College. 18 October 2006. Archived (PDF) from the original on 11 August 2020. Retrieved 17 February 2013.
^ Jump up to:a b Dumé, B. (23 April 2003). “Bismuth breaks half-life record for alpha decay”. Physicsworld.com. Bristol, England: Institute of Physics. Archived from the original on 13 December 2017. Retrieved 14 July 2015.
^ Jump up to:a b de Marcillac, P.; Coron, N.; Dambier, G.; Leblanc, J.; Moalic, J-P (2003). “Experimental detection of alpha-particles from the radioactive decay of natural bismuth”. Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
^ Sanderson, K. (17 October 2006). “Heaviest element made – again”. News@nature. doi:10.1038/news061016-4. S2CID 121148847. Archived from the original on 16 May 2020. Retrieved 8 March 2007.
^ Jump up to:a b Schewe, P.; Stein, B. (17 October 2000). “Elements 116 and 118 Are Discovered”. Physics News Update. American Institute of Physics. Archived from the original on 1 January 2012. Retrieved 19 October 2006.
^ Glanz, J. (6 April 2010). “Scientists Discover Heavy New Element”. The New York Times. Archived from the original on 19 June 2017. Retrieved 15 February 2017.
^ Oganessian, Yu. Ts.; Abdullin, F. Sh.; Bailey, P. D.; Benker, D. E.; Bennett, M. E.; Dmitriev, S. N.; Ezold, J. G.; Hamilton, J. H.; Henderson, R. A.; Itkis, M. G.; Lobanov, Yu. V.; Mezentsev, A. N.; Moody, K. J.; Nelson, S. L.; Polyakov, A. N.; Porter, C. E.; Ramayya, A. V.; Riley, F. D.; Roberto, J. B.; Ryabinin, M. A.; Rykaczewski, K. P.; Sagaidak, R. N.; Shaughnessy, D. A.; Shirokovsky, I. V.; Stoyer, M. A.; Subbotin, V. G.; Sudowe, R.; Sukhov, A. M.; Tsyganov, Yu. S.; et al. (April 2010). “Synthesis of a New Element with Atomic Number Z=117”. Physical Review Letters. 104 (14): 142502. Bibcode:2010PhRvL.104n2502O. doi:10.1103/PhysRevLett.104.142502. PMID 20481935.
^ ![]() This article incorporates text from this source, which is in the public domain: “Technetium-99”. epa.gov. United States Environmental Protection Agency. Archived from the original on 1 September 2015. Retrieved 26 February 2013.
This article incorporates text from this source, which is in the public domain: “Technetium-99”. epa.gov. United States Environmental Protection Agency. Archived from the original on 1 September 2015. Retrieved 26 February 2013.
^ “Origins of Heavy Elements”. Harvard–Smithsonian Center for Astrophysics. Archived from the original on 25 September 2020. Retrieved 26 February 2013.
^ “Atomic Number and Mass Numbers”. ndt-ed.org. Archived from the original on 12 February 2014. Retrieved 17 February 2013.
^ periodic.lanl.gov. “Periodic Table of Elements: LANL Carbon”. Los Alamos National Laboratory. Archived from the original on 25 January 2021. Retrieved 17 February 2013.
^ Katsuya Yamada. “Atomic mass, isotopes, and mass number” (PDF). Los Angeles Pierce College. Archived from the original (PDF) on 11 January 2014.
^ “Pure element”. European Nuclear Society. Archived from the original on 13 June 2017. Retrieved 13 August 2013.
^ Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). “The NUBASE2016 evaluation of nuclear properties” (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
^ Meija, Juris; et al. (2016). “Atomic weights of the elements 2013 (IUPAC Technical Report)”. Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). “Standard atomic weights of the elements 2021 (IUPAC Technical Report)”. Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
^ Wilford, J.N. (14 January 1992). “Hubble Observations Bring Some Surprises”. The New York Times. Archived from the original on 5 March 2008. Retrieved 15 February 2017.
^ Wright, E. L. (12 September 2004). “Big Bang Nucleosynthesis”. UCLA, Division of Astronomy. Archived from the original on 13 January 2018. Retrieved 22 February 2007.
^ Wallerstein, George; Iben, Icko; Parker, Peter; Boesgaard, Ann; Hale, Gerald; Champagne, Arthur; Barnes, Charles; Käppeler, Franz; et al. (1999). “Synthesis of the elements in stars: forty years of progress” (PDF). Reviews of Modern Physics. 69 (4): 995–1084. Bibcode:1997RvMP…69..995W. doi:10.1103/RevModPhys.69.995. hdl:2152/61093. Archived from the original (PDF) on 28 September 2006.
^ Earnshaw, A.; Greenwood, N. (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann.
^ Croswell, Ken (1996). Alchemy of the Heavens. Anchor. ISBN 978-0-385-47214-2. Archived from the original on 13 May 2011. Retrieved 10 October 2007.
^ Nielsen, Forrest H. (1999). “Ultratrace minerals”. In Maurice E. Shils; James A. Olsen; Moshe Shine; A. Catharine Ross (eds.). Modern nutrition in health and disease. Baltimore: Lippincott Williams & Wilkins. pp. 283–303. hdl:10113/46493. ISBN 978-0683307696.
^ Szklarska D, Rzymski P (May 2019). “Is Lithium a Micronutrient? From Biological Activity and Epidemiological Observation to Food Fortification”. Biol Trace Elem Res. 189 (1): 18–27. doi:10.1007/s12011-018-1455-2. PMC 6443601. PMID 30066063.
^ Enderle J, Klink U, di Giuseppe R, Koch M, Seidel U, Weber K, Birringer M, Ratjen I, Rimbach G, Lieb W (August 2020). “Plasma Lithium Levels in a General Population: A Cross-Sectional Analysis of Metabolic and Dietary Correlates”. Nutrients. 12 (8): 2489. doi:10.3390/nu12082489. PMC 7468710. PMID 32824874.
^ McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG (June 2014). “Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture”. Cell. 157 (6): 1380–92. doi:10.1016/j.cell.2014.05.009. PMC 4144415. PMID 24906154.
^ Zoroddu, Maria Antonietta; Aaseth, Jan; Crisponi, Guido; Medici, Serenella; Peana, Massimiliano; Nurchi, Valeria Marina (2019). “The essential metals for humans: a brief overview”. Journal of Inorganic Biochemistry. 195: 120–129. doi:10.1016/j.jinorgbio.2019.03.013.
^ Remick, Kaleigh; Helmann, John D. (30 January 2023). “The Elements of Life: A Biocentric Tour of the Periodic Table”. Advances in Microbial Physiology. 82. PubMed Central: 1–127. doi:10.1016/bs.ampbs.2022.11.001. ISBN 978-0-443-19334-7. PMC 10727122. PMID 36948652.
^ Daumann, Lena J. (25 April 2019). “Essential and Ubiquitous: The Emergence of Lanthanide Metallobiochemistry”. Angewandte Chemie International Edition. doi:10.1002/anie.201904090. Retrieved 15 June 2019.
^ Plato (2008) [c. 360 BC]. Timaeus. Forgotten Books. p. 45. ISBN 978-1-60620-018-6. Archived from the original on 14 April 2021. Retrieved 9 November 2020.
^ Hillar, M. (2004). “The Problem of the Soul in Aristotle’s De anima”. NASA/WMAP. Archived from the original on 9 September 2006. Retrieved 10 August 2006.
^ Partington, J. R. (1937). A Short History of Chemistry. New York: Dover Publications. ISBN 978-0-486-65977-0.
^ Boyle 1661, p. 36.
^ Boyle 1661, p. 38.
^ Boyle 1661, p. 37.
^ Boyle 1661, pp. 37–38.
^ Boyle 1661, pp. 38–39.
^ Boyle 1661, p. 42.
^ Jump up to:a b Boyle 1661, p. 29.
^ Boyle 1661, p. 41.
^ Jump up to:a b Boyle 1661, p. 145.
^ Boyle 1661, pp. 40–41.
^ Boyle 1661, p. 46.
^ Watts, Isaac (1726) [1724]. Logick: Or, the right use of reason in the enquiry after truth, with a variety of rules to guard against error in the affairs of religion and human life, as well as in the sciences. Printed for John Clark and Richard Hett. pp. 13–15.
^ Lavoisier, A. L. (1790). Elements of chemistry translated by Robert Kerr. Edinburgh. pp. 175–176. ISBN 978-0-415-17914-0. Archived from the original on 14 April 2021. Retrieved 24 August 2020.
^ Lavoisier, Antoine (1790) [1789]. Elements of chemistry: In a new systematic order, containing all the modern discoveries: Illustrated with thirteen copperplates. Translated by Kerr, Robert. William Creech. pp. 175–176.
^ Transactinide-2 Archived 3 March 2016 at the Wayback Machine. www.kernchemie.de
^ “IUPAC Announces Start of the Name Approval Process for the Element of Atomic Number 112” (PDF). IUPAC. 20 July 2009. Archived (PDF) from the original on 13 March 2012. Retrieved 27 August 2009.
^ “IUPAC (International Union of Pure and Applied Chemistry): Element 112 is Named Copernicium”. IUPAC. 20 February 2010. Archived from the original on 24 February 2010.
^ Oganessian, Yu. Ts.; Utyonkov, V.; Lobanov, Yu.; Abdullin, F.; Polyakov, A.; Sagaidak, R.; Shirokovsky, I.; Tsyganov, Yu.; et al. (2006). “Evidence for Dark Matter” (PDF). Physical Review C. 74 (4): 044602. Bibcode:2006PhRvC..74d4602O. doi:10.1103/PhysRevC.74.044602. Archived (PDF) from the original on 13 February 2021. Retrieved 8 October 2007.
^ Greiner, W. “Recommendations” (PDF). 31st meeting, PAC for Nuclear Physics. Joint Institute for Nuclear Research. Archived from the original (PDF) on 14 April 2010.
^ Staff (30 November 2016). “IUPAC Announces the Names of the Elements 113, 115, 117, and 118”. IUPAC. Archived from the original on 29 July 2018. Retrieved 1 December 2016.
^ St. Fleur, Nicholas (1 December 2016). “Four New Names Officially Added to the Periodic Table of Elements”. The New York Times. Archived from the original on 1 January 2022. Retrieved 1 December 2016.
^ “Periodic Table – Royal Society of Chemistry”. www.rsc.org.
^ “Online Etymology Dictionary”. etymonline.com.
^ “beryl”. Merriam-Webster. Archived from the original on 9 October 2013. Retrieved 27 January 2014.
^ van der Krogt, Peter. “Wolframium Wolfram Tungsten”. Elementymology & Elements Multidict. Archived from the original on 23 January 2010. Retrieved 11 March 2010.
^ Originally assessed as 0.7 by Pauling but never revised after other elements’ electronegativities were updated for precision. Predicted to be higher than that of caesium.
^ Konings, Rudy J. M.; Beneš, Ondrej. “The Thermodynamic Properties of the 𝑓-Elements and Their Compounds. I. The Lanthanide and Actinide Metals”. Journal of Physical and Chemical Reference Data. doi:10.1063/1.3474238.