
Structure of Atom ?
The structure of an atom refers to the arrangement of its fundamental particles: protons, neutrons, and electrons. Here’s a detailed explanation:
1. Nucleus:
- Location: At the center of the atom.
- Contains:
- Protons: Positively charged particles.
- Neutrons: Neutral particles with no charge.
- Mass: The nucleus accounts for almost all the mass of the atom since protons and neutrons are much heavier than electrons.
2. Electrons:
- Location: Revolve around the nucleus in specific regions called energy levels or shells.
- Charge: Negative.
- Mass: Extremely small compared to protons and neutrons.
3. Subatomic Particles:
- Protons:
- Mass: ~1 atomic mass unit (amu).
- Charge: +1.
- Neutrons:
- Mass: ~1 amu (slightly heavier than protons).
- Charge: Neutral (0).
- Electrons:
- Mass: ~1/1836 of a proton.
- Charge: -1.
4. Atomic Number (Z):
- Represents the number of protons in the nucleus.
- Determines the identity of the element.
5. Mass Number (A):
- Sum of protons and neutrons in the nucleus.
- Mass Number (A)=Protons+Neutrons\text{Mass Number (A)} = \text{Protons} + \text{Neutrons}Mass Number (A)=Protons+Neutrons.
6. Electron Shells and Configuration:
- Electrons occupy shells or energy levels labeled as K, L, M, N, etc.
- The number of electrons in each shell is determined by 2n22n^22n2, where nnn is the shell number (e.g., K = 1, L = 2).
7. Neutral Atom:
- The number of protons equals the number of electrons, making the atom electrically neutral.
Example:
For a carbon atom:
- Atomic Number (Z): 6 (6 protons, 6 electrons).
- Mass Number (A): 12 (6 protons + 6 neutrons).
Would you like a diagram or more details on atomic models (like Bohr’s model or quantum theory)?
What is Structure of Atom ?
The structure of an atom is a scientific concept that describes how an atom is composed and how its parts are arranged. An atom is the smallest unit of matter that retains the properties of an element. It consists of three main types of subatomic particles: protons, neutrons, and electrons.
Components of an Atom:
1. Nucleus:
- Location: The dense central core of the atom.
- Composition:
- Protons: Positively charged particles.
- Neutrons: Neutral particles with no charge.
- Properties:
- Contains almost all the mass of the atom.
- Held together by the strong nuclear force.
2. Electrons:
- Location: Surround the nucleus in regions called energy levels or electron clouds.
- Charge: Negative (-).
- Mass: Negligible compared to protons and neutrons.
- Behavior:
- Electrons are distributed in discrete energy levels or shells.
- The arrangement of electrons determines an atom’s chemical properties.
Subatomic Particles:
| Particle | Charge | Mass | Location |
|---|---|---|---|
| Proton | Positive (+1) | ~1 atomic mass unit | Nucleus |
| Neutron | Neutral (0) | ~1 atomic mass unit | Nucleus |
| Electron | Negative (-1) | ~1/1836 of a proton | Outside nucleus |
Key Atomic Characteristics:
- Atomic Number (Z):
- Equals the number of protons in the nucleus.
- Determines the element’s identity (e.g., Hydrogen: Z = 1, Carbon: Z = 6).
- Mass Number (A):
- Equals the sum of protons and neutrons in the nucleus.
- A=Protons+NeutronsA = \text{Protons} + \text{Neutrons}A=Protons+Neutrons.
- Electron Configuration:
- Electrons occupy specific energy levels around the nucleus.
- These levels are labeled K, L, M, N, etc.
- The distribution of electrons follows the rule 2n22n^22n2, where nnn is the shell number.
Atomic Models:
Over time, several models have been proposed to explain the structure of the atom:
- Dalton’s Model: Atoms are indivisible spheres.
- Thomson’s Model: Plum pudding model with electrons embedded in a positive sphere.
- Rutherford’s Model: Atom has a dense nucleus with electrons orbiting around it.
- Bohr’s Model: Electrons revolve in fixed orbits with quantized energy levels.
- Quantum Mechanical Model: Describes electrons in terms of probabilities within orbitals.
Would you like a specific atomic model or a diagram to better visualize this?
Who is required Structure of Atom ?
The structure of an atom is essential knowledge in various fields and for different groups of people. Here’s who typically requires this knowledge and why:
1. Students and Educators:
- Why:
- Fundamental concept in physics, chemistry, and biology.
- Forms the basis for understanding elements, compounds, and reactions.
- Who:
- School students (science curriculum).
- University students (chemistry, material science, physics, and related disciplines).
2. Scientists and Researchers:
- Why:
- Helps in studying the properties of elements and developing new materials.
- Essential for advancements in nanotechnology, pharmaceuticals, and quantum mechanics.
- Who:
- Chemists, physicists, and materials scientists.
- Biophysicists and biochemists.
3. Engineers:
- Why:
- Understanding atomic interactions is crucial for fields like materials engineering, electronics, and nuclear engineering.
- Used in developing semiconductors, alloys, and nuclear reactors.
- Who:
- Chemical engineers, electrical engineers, and mechanical engineers.
4. Medical Professionals and Pharmacists:
- Why:
- Knowledge of atoms and molecules is critical for understanding biochemical reactions.
- Forms the basis for designing drugs and medical imaging technologies.
- Who:
- Pharmacists, radiologists, and medical researchers.
5. Environmental Scientists:
- Why:
- Atomic structure helps explain processes like pollution, climate change, and natural resource management.
- Who:
- Environmental chemists and ecologists.
6. Industry Professionals:
- Why:
- Helps in manufacturing products like batteries, plastics, fuels, and electronics.
- Who:
- Industrial chemists, metallurgists, and energy sector professionals.
7. General Knowledge Enthusiasts:
- Why:
- Understanding the atomic structure provides insight into how the universe and matter are constructed.
Would you like to explore how the atomic structure applies to a specific field?
When is required Structure of Atom ?
The structure of an atom is required in various contexts across academic, professional, and real-world applications. Here’s when and why it’s needed:
1. In Education:
- When:
- During school and university science classes (physics, chemistry, and biology).
- While learning about chemical bonding, periodic table trends, and reactions.
- Why:
- To build foundational knowledge in science and understand the nature of matter.
2. In Research and Development:
- When:
- Developing new materials, drugs, or technologies.
- Conducting experiments in fields like nanotechnology, quantum physics, and biotechnology.
- Why:
- To study the behavior and properties of elements and compounds at the atomic level.
3. In Industrial Applications:
- When:
- Designing and improving products like semiconductors, batteries, and pharmaceuticals.
- Optimizing processes in industries like metallurgy, petrochemicals, and electronics.
- Why:
- Understanding atomic interactions is crucial for innovation and quality control.
4. In Medical Applications:
- When:
- Developing diagnostic tools like X-rays, MRI, and PET scans.
- Researching drugs and understanding biochemical reactions.
- Why:
- Atomic knowledge underpins imaging technologies and molecular medicine.
5. In Environmental Science:
- When:
- Studying pollution, climate change, or the effects of radiation.
- Managing resources like water, air, and soil.
- Why:
- To understand the interaction of elements and compounds in ecosystems.
6. In Everyday Life:
- When:
- Explaining phenomena like electricity, heat, or magnetism.
- Discussing topics like radiation safety, renewable energy, or the chemical properties of materials.
- Why:
- Atomic knowledge helps explain the basic principles of the physical world.
7. In Examinations and Certifications:
- When:
- Appearing for science-related exams (academic or competitive).
- Pursuing professional certifications in engineering, medicine, or environmental science.
- Why:
- Many questions test the understanding of atomic structure and its applications.
Would you like to know specific examples or applications where atomic structure plays a critical role?
Where is required Structure of Atom ?
The structure of an atom is required in a wide range of fields and places where the understanding of matter, energy, and their interactions is essential. Here’s where it is most relevant:
1. Educational Institutions:
- Schools and Colleges:
- Teaching basic science concepts in physics, chemistry, and biology.
- Universities and Research Labs:
- Advanced studies in materials science, quantum mechanics, and molecular biology.
2. Scientific Research Facilities:
- Where:
- Laboratories focused on particle physics, nanotechnology, and spectroscopy.
- Research centers studying nuclear energy or fundamental atomic theories.
- Why:
- To investigate atomic and molecular behavior for scientific advancements.
3. Industries and Manufacturing Units:
- Chemical Industry:
- For chemical reactions, material synthesis, and product development.
- Electronics and Semiconductor Industry:
- Designing microchips and understanding the behavior of electrons in materials.
- Energy Sector:
- In nuclear power plants for understanding fission and fusion reactions.
- Pharmaceutical Industry:
- For drug design and understanding biochemical interactions.
4. Medical Field:
- Where:
- Hospitals and diagnostic centers using X-rays, MRIs, and CT scans.
- Research labs developing new medical treatments and imaging technologies.
- Why:
- Understanding atomic interactions is vital for diagnostics and drug discovery.
5. Environmental Science and Resource Management:
- Where:
- Pollution control facilities and renewable energy projects.
- Climate research centers studying atomic and molecular effects on the environment.
- Why:
- To understand how atoms and molecules interact with ecosystems.
6. Technology and Innovation Hubs:
- Where:
- Artificial intelligence and quantum computing labs.
- Advanced material design centers (e.g., for graphene, superconductors).
- Why:
- Knowledge of atomic structure drives technological progress.
7. Space and Astronomy Research:
- Where:
- Observatories studying atomic behavior in stars and cosmic phenomena.
- Space agencies (e.g., NASA, ESA) analyzing the composition of extraterrestrial materials.
- Why:
- Atomic structure is key to understanding the universe’s building blocks.
8. Government and Regulatory Bodies:
- Where:
- Organizations setting safety standards for radiation and nuclear energy.
- Institutions managing the use of isotopes in agriculture and healthcare.
- Why:
- To ensure public safety and effective resource utilization.
Would you like details about any specific application or context?
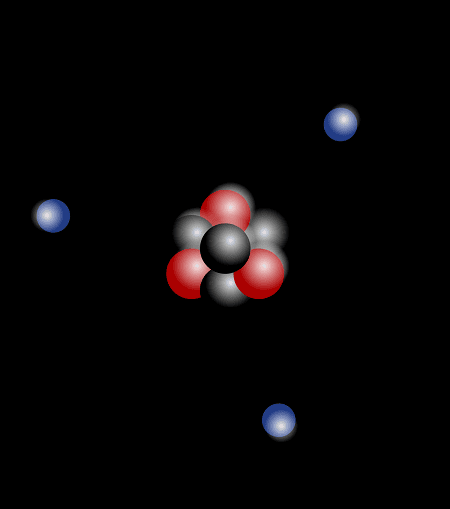
How is required Structure of Atom ?
The structure of an atom is required in a systematic and scientific way to understand, analyze, and apply the principles of atomic and molecular interactions. Here’s how it is required in various contexts:
1. In Education:
- How:
- Taught through atomic models like Dalton’s, Thomson’s, Rutherford’s, and Bohr’s models.
- Integrated into curriculums to explain chemical reactions, periodic table trends, and molecular interactions.
- Supported by experiments (e.g., Rutherford’s gold foil experiment) to demonstrate atomic properties.
- Purpose:
- To build foundational knowledge for students in science disciplines.
2. In Research:
- How:
- Researchers use the atomic structure to predict material behavior at a microscopic level.
- Quantum mechanics and spectroscopy tools (e.g., NMR, X-ray diffraction) are employed to analyze atomic arrangements.
- Purpose:
- To innovate in materials science, nanotechnology, and medicine.
3. In Industry:
- How:
- Chemical engineers design processes based on the reactivity of atoms and molecules.
- Semiconductor industries use principles like electron configurations and energy bands.
- Nuclear industries apply concepts of atomic fission and fusion.
- Purpose:
- To develop efficient technologies and products.
4. In Medicine:
- How:
- Radiologists rely on the atomic behavior of isotopes for imaging and treatment.
- Pharmacologists use molecular interactions for drug design.
- Purpose:
- To improve diagnostics and therapeutic solutions.
5. In Environmental Science:
- How:
- Atomic structure helps explain pollutant behavior and energy transformations.
- Used to design renewable energy solutions, like solar cells.
- Purpose:
- To solve environmental challenges and promote sustainability.
6. In Advanced Technologies:
- How:
- Quantum computing depends on understanding atomic and subatomic particles.
- Nanotechnology uses atomic arrangements to create novel materials.
- Purpose:
- To push the boundaries of innovation.
7. For Public Safety and Regulation:
- How:
- Governments use atomic principles for radiation safety standards.
- Atomic properties are critical in managing nuclear energy and waste.
- Purpose:
- To ensure safety and compliance in industrial and healthcare applications.
Would you like more detailed examples or practical applications?
White paper on is Structure of Atom ?
A white paper on the structure of an atom would serve as a comprehensive document aimed at educating or informing readers about the atom’s fundamental components, history, applications, and significance in science and technology. Below is an outline for creating such a white paper:
White Paper on the Structure of an Atom
1. Executive Summary
- Brief overview of what an atom is.
- Importance of understanding atomic structure in science and technology.
- Key takeaways for readers.
2. Introduction
- Definition of an atom: The smallest unit of matter that retains the properties of an element.
- Historical context:
- Democritus’ atomic theory (philosophical idea).
- Modern scientific discoveries by Dalton, Thomson, Rutherford, and Bohr.
- Purpose of the white paper: Explain the structure of an atom and its implications.
3. Components of an Atom
- Nucleus:
- Protons (positive charge, mass ~1 atomic unit).
- Neutrons (neutral charge, mass ~1 atomic unit).
- Electrons:
- Negatively charged, orbiting the nucleus.
- Distributed in energy levels or shells.
4. Atomic Models
- Dalton’s Atomic Theory: Early model emphasizing indivisible atoms.
- Thomson’s Plum Pudding Model: Electrons embedded in a positive sphere.
- Rutherford’s Nuclear Model: Dense, positively charged nucleus with orbiting electrons.
- Bohr’s Model: Electrons in discrete orbits with quantized energy.
- Quantum Mechanical Model: Electrons as probability clouds in orbitals.
5. Key Concepts
- Atomic Number (Z): Number of protons in the nucleus.
- Mass Number (A): Sum of protons and neutrons.
- Isotopes: Variants of elements with different neutron counts.
- Electron Configuration: Distribution of electrons in energy levels.
6. Applications of Atomic Structure
- Chemistry:
- Understanding reactions and bonding.
- Predicting periodic table trends.
- Physics:
- Basis for quantum mechanics and particle physics.
- Medicine:
- Imaging technologies (X-rays, MRIs) and radiotherapy.
- Energy:
- Nuclear fission and fusion for power generation.
- Technology:
- Development of semiconductors and nanotechnology.
7. Role in Modern Science and Technology
- Contributions to advancements in material science, quantum computing, and renewable energy.
- Impact on understanding the universe through astrophysics and cosmology.
8. Challenges and Future Directions
- Challenges in manipulating atomic structures (e.g., quantum mechanics complexities).
- Future opportunities in harnessing atomic properties for innovation (e.g., quantum computing, fusion energy).
9. Conclusion
- Recap of the importance of atomic structure in science and technology.
- Encouragement for further research and education on the topic.
10. References and Resources
- Citing key research papers, textbooks, and online resources for further reading.
Would you like me to expand on any specific section or assist in drafting a detailed white paper?
Industrial application of Structure of Atom ?
The structure of an atom is foundational to many industries, as it explains the behavior of matter at the most fundamental level. Understanding atomic structure enables the design and optimization of processes, materials, and technologies. Below are some key industrial applications:
1. Chemical Industry
- Applications:
- Designing chemical reactions based on atomic and molecular interactions.
- Synthesis of new compounds and materials.
- Catalysis in chemical processes.
- Examples:
- Production of fertilizers, plastics, and pharmaceuticals.
- Refining crude oil into usable fuels.
2. Electronics and Semiconductor Industry
- Applications:
- Development of semiconductors based on electron behavior in atoms.
- Miniaturization of transistors in integrated circuits.
- Examples:
- Manufacture of microchips, LEDs, and solar cells.
3. Energy Sector
- Applications:
- Nuclear power generation using principles of atomic fission and fusion.
- Design of batteries based on electron transfer and energy storage.
- Examples:
- Uranium enrichment for nuclear reactors.
- Lithium-ion batteries for electric vehicles.
4. Metallurgy and Material Science
- Applications:
- Understanding the arrangement of atoms in metals and alloys.
- Improving material properties like strength, ductility, and corrosion resistance.
- Examples:
- Steel production with controlled atomic composition.
- Development of lightweight materials for aerospace.
5. Pharmaceutical and Biotechnology Industry
- Applications:
- Drug design based on molecular interactions at the atomic level.
- Development of radiopharmaceuticals for diagnostics and therapy.
- Examples:
- Molecular modeling for new medications.
- Use of isotopes in cancer treatments.
6. Nanotechnology
- Applications:
- Manipulating atomic and molecular structures to create nanomaterials.
- Designing nanoparticles for specific industrial or medical uses.
- Examples:
- Carbon nanotubes for stronger, lighter materials.
- Gold nanoparticles in medical imaging.
7. Environmental Applications
- Applications:
- Understanding atomic interactions in pollution control.
- Designing catalysts for cleaner industrial processes.
- Examples:
- Catalytic converters in vehicles to reduce emissions.
- Water purification technologies using atomic-scale filtration.
8. Agriculture
- Applications:
- Use of isotopes to study nutrient absorption in plants.
- Development of fertilizers based on atomic and molecular insights.
- Examples:
- Tracing soil nutrient uptake using radiolabeled isotopes.
9. Aerospace and Defense
- Applications:
- Development of heat-resistant and lightweight materials.
- Use of atomic principles in GPS and satellite technologies.
- Examples:
- Alloy development for spacecraft.
- Nuclear-powered submarines.
10. Food and Beverage Industry
- Applications:
- Preservation techniques using radiation.
- Analyzing food quality at the atomic level.
- Examples:
- Food irradiation to extend shelf life.
- Spectroscopic analysis for quality control.
11. Medical Technology
- Applications:
- Atomic interactions in diagnostic imaging (MRI, CT scans).
- Radiation therapy for cancer treatment.
- Examples:
- Use of iodine isotopes in thyroid treatment.
- X-ray machines based on electron transitions.
12. Renewable Energy
- Applications:
- Solar cells designed using knowledge of electron excitation.
- Hydrogen fuel cells based on atomic-level energy release.
- Examples:
- Photovoltaic panels.
- Electrolysis for hydrogen production.
Would you like a detailed explanation of any specific industry’s application?
Research and development of Structure of Atom?
Research and Development (R&D) in the Structure of Atom
Research and development in the structure of an atom have paved the way for revolutionary advancements across multiple disciplines. This field focuses on understanding atomic and subatomic particles, their interactions, and how this knowledge can be applied to science, technology, and industry.
Key Areas of R&D in Atomic Structure
1. Fundamental Research
- Objective: To deepen understanding of atomic and subatomic particles.
- Topics:
- Study of quantum mechanics and electron behavior.
- Exploration of atomic nuclei, isotopes, and radioactivity.
- Advances in the Standard Model of particle physics.
- Significance: Provides the theoretical foundation for applied sciences.
2. Materials Science
- Objective: To design and develop materials with enhanced properties.
- Topics:
- Atomic-level manipulation to create alloys, polymers, and nanomaterials.
- Investigation of crystal structures and atomic bonding.
- Applications: Aerospace, electronics, construction, and healthcare industries.
3. Nuclear Energy and Fusion Research
- Objective: Harness atomic nuclei for energy production.
- Topics:
- Efficient nuclear fission processes.
- Development of sustainable nuclear fusion reactors (e.g., ITER project).
- Applications: Clean and efficient energy sources.
4. Nanotechnology
- Objective: Manipulate matter at the atomic scale to create nanostructures.
- Topics:
- Design of nanoparticles for drug delivery, imaging, and cancer treatment.
- Research on nanowires, quantum dots, and other nanoscale materials.
- Applications: Medicine, electronics, and environmental solutions.
5. Quantum Computing
- Objective: Exploit quantum states of atoms and particles for computation.
- Topics:
- Research on qubits, atomic-level data storage, and quantum entanglement.
- Development of quantum algorithms for complex problem-solving.
- Applications: Cryptography, machine learning, and large-scale simulations.
6. Spectroscopy and Imaging
- Objective: Develop techniques to observe atomic and molecular structures.
- Topics:
- Research on X-ray, electron microscopy, and neutron diffraction methods.
- Advancing Nuclear Magnetic Resonance (NMR) and Mass Spectrometry.
- Applications: Material characterization, pharmaceuticals, and quality control.
7. Particle Physics and High-Energy Research
- Objective: Explore subatomic particles and their interactions.
- Topics:
- Experiments in particle accelerators like CERN’s Large Hadron Collider (LHC).
- Discovery of particles like the Higgs boson and their role in atomic structure.
- Applications: Fundamental science, astrophysics, and cosmology.
Emerging Trends in Atomic R&D
- Artificial Intelligence in Atomic Simulations:
- Use AI to predict atomic behaviors and model complex systems.
- Green Chemistry:
- Atom-efficient reactions to minimize waste in chemical processes.
- Advanced Computational Techniques:
- Quantum simulations to solve problems in atomic and molecular physics.
- Sustainable Nuclear Fusion:
- Research on magnetic confinement and inertial fusion for energy generation.
Applications of R&D
- Healthcare: Precision medicine, radiopharmaceuticals, and diagnostic imaging.
- Environment: Carbon capture, pollution monitoring, and sustainable energy.
- Technology: Miniaturized electronics, sensors, and superconductors.
- Defense: Development of nuclear technologies and advanced materials.
Future Directions
- Improved understanding of dark matter and its relation to atomic structure.
- Development of room-temperature superconductors for energy efficiency.
- Expanding quantum computing to practical, large-scale applications.
- Advancing sustainable energy solutions through nuclear fusion.
Would you like to explore any specific topic or application of atomic R&D in detail?
Courtesy : Whats Up Dude
References
- ^ “DOE Explains…Nuclei”. Energy.gov. Retrieved 5 November 2024.
- ^ Pullman, Bernard (1998). The Atom in the History of Human Thought. Oxford, England: Oxford University Press. pp. 31–33. ISBN 978-0-19-515040-7. Archived from the original on 5 February 2021. Retrieved 25 October 2020.
- ^ Melsen (1952). From Atomos to Atom, pp. 18–19
- ^ Pullman (1998). The Atom in the History of Human Thought, p. 201
- ^ Pullman (1998). The Atom in the History of Human Thought, p. 199: “The constant ratios, expressible in terms of integers, of the weights of the constituents in composite bodies could be construed as evidence on a macroscopic scale of interactions at the microscopic level between basic units with fixed weights. For Dalton, this agreement strongly suggested a corpuscular structure of matter, even though it did not constitute definite proof.”
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 36
- ^ Melsen (1952). From Atomos to Atom, p. 137
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 28
- ^ Millington (1906). John Dalton, p. 113
- ^ Dalton (1808). A New System of Chemical Philosophy vol. 1, pp. 316–319
- ^ Holbrow et al. (2010). Modern Introductory Physics, pp. 65–66
- ^ J. J. Thomson (1897). “Cathode rays”. Philosophical Magazine. 44 (269): 293–316.
- ^ In his book The Corpuscular Theory of Matter (1907), Thomson estimates electrons to be 1/1700 the mass of hydrogen.
- ^ “The Mechanism Of Conduction In Metals” Archived 25 October 2012 at the Wayback Machine, Think Quest.
- ^ Thomson, J.J. (August 1901). “On bodies smaller than atoms”. The Popular Science Monthly: 323–335. Archived from the original on 1 December 2016. Retrieved 21 June 2009.
- ^ J. J. Thomson (1907). On the Corpuscular Theory of Matter, p. 26: “The simplest interpretation of these results is that the positive ions are the atoms or groups of atoms of various elements from which one or more corpuscles have been removed […] while the negative electrified body is one with more corpuscles than the unelectrified one.”
- ^ J. J. Thomson (1907). The Corpuscular Theory of Matter, p. 103: “In default of exact knowledge of the nature of the way in which positive electricity occurs in the atom, we shall consider a case in which the positive electricity is distributed in the way most amenable to mathematical calculation, i.e., when it occurs as a sphere of uniform density, throughout which the corpuscles are distributed.”
- ^ Giora Hon; Bernard R. Goldstein (6 September 2013). “J. J. Thomson’s plum-pudding atomic model: The making of a scientific myth”. Annalen der Physik. 525 (8–9): A129–A133. Bibcode:2013AnP…525A.129H. doi:10.1002/andp.201300732. ISSN 0003-3804.
- ^ Heilbron (2003). Ernest Rutherford and the Explosion of Atoms, pp. 64–68
- ^ Stern, David P. (16 May 2005). “The Atomic Nucleus and Bohr’s Early Model of the Atom”. NASA/Goddard Space Flight Center. Archived from the original on 20 August 2007.
- ^ Bohr, Niels (11 December 1922). “Niels Bohr, The Nobel Prize in Physics 1922, Nobel Lecture”. Nobel Foundation. Archived from the original on 15 April 2008.
- ^ J. J. Thomson (1898). “On the Charge of Electricity carried by the Ions produced by Röntgen Rays”. The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science. 5. 46 (283): 528–545. doi:10.1080/14786449808621229.
- ^ J. J. Thomson (1907). The Corpuscular Theory of Matter. p. 26–27: “In an unelectrified atom there are as many units of positive electricity as there are of negative; an atom with a unit of positive charge is a neutral atom which has lost one corpuscle, while an atom with a unit of negative charge is a neutral atom to which an additional corpuscle has been attached.”
- ^ Rutherford, Ernest (1919). “Collisions of alpha Particles with Light Atoms. IV. An Anomalous Effect in Nitrogen”. Philosophical Magazine. 37 (222): 581. doi:10.1080/14786440608635919.
- ^ The Development of the Theory of Atomic Structure (Rutherford 1936). Reprinted in Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936:
“In 1919 I showed that when light atoms were bombarded by α-particles they could be broken up with the emission of a proton, or hydrogen nucleus. We therefore presumed that a proton must be one of the units of which the nuclei of other atoms were composed…” - ^ Orme Masson (1921). “The Constitution of Atoms”. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 41 (242): 281–285. doi:10.1080/14786442108636219.
Footnote by Ernest Rutherford: ‘At the time of writing this paper in Australia, Professor Orme Masson was not aware that the name “proton” had already been suggested as a suitable name for the unit of mass nearly 1, in terms of oxygen 16, that appears to enter into the nuclear structure of atoms. The question of a suitable name for this unit was discussed at an informal meeting of a number of members of Section A of the British Association at Cardiff this year. The name “baron” suggested by Professor Masson was mentioned, but was considered unsuitable on account of the existing variety of meanings. Finally the name “proton” met with general approval, particularly as it suggests the original term “protyle ” given by Prout in his well-known hypothesis that all atoms are built up of hydrogen. The need of a special name for the nuclear unit of mass 1 was drawn attention to by Sir Oliver Lodge at the Sectional meeting, and the writer then suggested the name “proton.”‘ - ^ Eric Scerri (2020). The Periodic Table: Its Story and Its Significance, p. 185
- ^ Helge Kragh (2012). Niels Bohr and the Quantum Atom, p. 33
- ^ James Chadwick (1932). “Possible Existence of a Neutron” (PDF). Nature. 129 (3252): 312. Bibcode:1932Natur.129Q.312C. doi:10.1038/129312a0. S2CID 4076465. Archived (PDF) from the original on 9 October 2022.
- ^ Jump up to:a b Pais, Abraham (1986). Inward Bound: Of Matter and Forces in the Physical World. New York: Oxford University Press. pp. 228–230. ISBN 978-0-19-851971-3.
- ^ McEvoy, J. P.; Zarate, Oscar (2004). Introducing Quantum Theory. Totem Books. pp. 110–114. ISBN 978-1-84046-577-8.
- ^ Kozłowski, Miroslaw (2019). “The Schrödinger equation A History”.
- ^ Chad Orzel (16 September 2014). “What is the Heisenberg Uncertainty Principle?”. TED-Ed. Archived from the original on 13 September 2015 – via YouTube.
- ^ Brown, Kevin (2007). “The Hydrogen Atom”. MathPages. Archived from the original on 5 September 2012.
- ^ Harrison, David M. (2000). “The Development of Quantum Mechanics”. University of Toronto. Archived from the original on 25 December 2007.
- ^ Demtröder, Wolfgang (2002). Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics (1st ed.). Springer. pp. 39–42. ISBN 978-3-540-20631-6. OCLC 181435713.
- ^ Woan, Graham (2000). The Cambridge Handbook of Physics. Cambridge University Press. p. 8. ISBN 978-0-521-57507-2. OCLC 224032426.
- ^ Mohr, P.J.; Taylor, B.N. and Newell, D.B. (2014), “The 2014 CODATA Recommended Values of the Fundamental Physical Constants” Archived 11 February 2012 at the Wayback Machine (Web Version 7.0). The database was developed by J. Baker, M. Douma, and S. Kotochigova. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899.
- ^ MacGregor, Malcolm H. (1992). The Enigmatic Electron. Oxford University Press. pp. 33–37. ISBN 978-0-19-521833-6. OCLC 223372888.
- ^ Jump up to:a b Particle Data Group (2002). “The Particle Adventure”. Lawrence Berkeley Laboratory. Archived from the original on 4 January 2007.
- ^ Jump up to:a b Schombert, James (18 April 2006). “Elementary Particles”. University of Oregon. Archived from the original on 30 August 2011.
- ^ Jevremovic, Tatjana (2005). Nuclear Principles in Engineering. Springer. p. 63. ISBN 978-0-387-23284-3. OCLC 228384008.
- ^ Pfeffer, Jeremy I.; Nir, Shlomo (2000). Modern Physics: An Introductory Text. Imperial College Press. pp. 330–336. ISBN 978-1-86094-250-1. OCLC 45900880.
- ^ Wenner, Jennifer M. (10 October 2007). “How Does Radioactive Decay Work?”. Carleton College. Archived from the original on 11 May 2008.
- ^ Jump up to:a b c Raymond, David (7 April 2006). “Nuclear Binding Energies”. New Mexico Tech. Archived from the original on 1 December 2002.
- ^ Mihos, Chris (23 July 2002). “Overcoming the Coulomb Barrier”. Case Western Reserve University. Archived from the original on 12 September 2006.
- ^ Staff (30 March 2007). “ABC’s of Nuclear Science”. Lawrence Berkeley National Laboratory. Archived from the original on 5 December 2006.
- ^ Makhijani, Arjun; Saleska, Scott (2 March 2001). “Basics of Nuclear Physics and Fission”. Institute for Energy and Environmental Research. Archived from the original on 16 January 2007.
- ^ Shultis, J. Kenneth; Faw, Richard E. (2002). Fundamentals of Nuclear Science and Engineering. CRC Press. pp. 10–17. ISBN 978-0-8247-0834-4. OCLC 123346507.
- ^ Fewell, M.P. (1995). “The atomic nuclide with the highest mean binding energy”. American Journal of Physics. 63 (7): 653–658. Bibcode:1995AmJPh..63..653F. doi:10.1119/1.17828.
- ^ Mulliken, Robert S. (1967). “Spectroscopy, Molecular Orbitals, and Chemical Bonding”. Science. 157 (3784): 13–24. Bibcode:1967Sci…157…13M. doi:10.1126/science.157.3784.13. PMID 5338306.
- ^ Jump up to:a b Brucat, Philip J. (2008). “The Quantum Atom”. University of Florida. Archived from the original on 7 December 2006.
- ^ Manthey, David (2001). “Atomic Orbitals”. Orbital Central. Archived from the original on 10 January 2008.
- ^ Herter, Terry (2006). “Lecture 8: The Hydrogen Atom”. Cornell University. Archived from the original on 22 February 2012.
- ^ Bell, R.E.; Elliott, L.G. (1950). “Gamma-Rays from the Reaction H1(n,γ)D2 and the Binding Energy of the Deuteron”. Physical Review. 79 (2): 282–285. Bibcode:1950PhRv…79..282B. doi:10.1103/PhysRev.79.282.
- ^ Smirnov, Boris M. (2003). Physics of Atoms and Ions. Springer. pp. 249–272. ISBN 978-0-387-95550-6.
- ^ Matis, Howard S. (9 August 2000). “The Isotopes of Hydrogen”. Guide to the Nuclear Wall Chart. Lawrence Berkeley National Lab. Archived from the original on 18 December 2007.
- ^ Weiss, Rick (17 October 2006). “Scientists Announce Creation of Atomic Element, the Heaviest Yet”. Washington Post. Archived from the original on 20 August 2011.
- ^ Jump up to:a b Sills, Alan D. (2003). Earth Science the Easy Way. Barron’s Educational Series. pp. 131–134. ISBN 978-0-7641-2146-3. OCLC 51543743.
- ^ Dumé, Belle (23 April 2003). “Bismuth breaks half-life record for alpha decay”. Physics World. Archived from the original on 14 December 2007.
- ^ Lindsay, Don (30 July 2000). “Radioactives Missing From The Earth”. Don Lindsay Archive. Archived from the original on 28 April 2007.
- ^ Tuli, Jagdish K. (April 2005). “Nuclear Wallet Cards”. National Nuclear Data Center, Brookhaven National Laboratory. Archived from the original on 3 October 2011.
- ^ CRC Handbook (2002).
- ^ Krane, K. (1988). Introductory Nuclear Physics. John Wiley & Sons. pp. 68. ISBN 978-0-471-85914-7.
- ^ Jump up to:a b Mills, Ian; Cvitaš, Tomislav; Homann, Klaus; Kallay, Nikola; Kuchitsu, Kozo (1993). Quantities, Units and Symbols in Physical Chemistry (2nd ed.). Oxford: International Union of Pure and Applied Chemistry, Commission on Physiochemical Symbols Terminology and Units, Blackwell Scientific Publications. p. 70. ISBN 978-0-632-03583-0. OCLC 27011505.
- ^ Chieh, Chung (22 January 2001). “Nuclide Stability”. University of Waterloo. Archived from the original on 30 August 2007.
- ^ “Atomic Weights and Isotopic Compositions for All Elements”. National Institute of Standards and Technology. Archived from the original on 31 December 2006. Retrieved 4 January 2007.
- ^ Audi, G.; Wapstra, A.H.; Thibault, C. (2003). “The Ame2003 atomic mass evaluation (II)” (PDF). Nuclear Physics A. 729 (1): 337–676. Bibcode:2003NuPhA.729..337A. doi:10.1016/j.nuclphysa.2003.11.003. Archived (PDF) from the original on 16 October 2005.
- ^ Ghosh, D.C.; Biswas, R. (2002). “Theoretical calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii”. Int. J. Mol. Sci. 3 (11): 87–113. doi:10.3390/i3020087.
- ^ Shannon, R.D. (1976). “Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides” (PDF). Acta Crystallographica A. 32 (5): 751–767. Bibcode:1976AcCrA..32..751S. doi:10.1107/S0567739476001551. Archived (PDF) from the original on 14 August 2020. Retrieved 25 August 2019.
- ^ Dong, Judy (1998). “Diameter of an Atom”. The Physics Factbook. Archived from the original on 4 November 2007.
- ^ Zumdahl, Steven S. (2002). Introductory Chemistry: A Foundation (5th ed.). Houghton Mifflin. ISBN 978-0-618-34342-3. OCLC 173081482. Archived from the original on 4 March 2008.
- ^ Bethe, Hans (1929). “Termaufspaltung in Kristallen”. Annalen der Physik. 3 (2): 133–208. Bibcode:1929AnP…395..133B. doi:10.1002/andp.19293950202.
- ^ Birkholz, Mario (1995). “Crystal-field induced dipoles in heteropolar crystals – I. concept”. Z. Phys. B. 96 (3): 325–332. Bibcode:1995ZPhyB..96..325B. CiteSeerX 10.1.1.424.5632. doi:10.1007/BF01313054. S2CID 122527743.
- ^ Birkholz, M.; Rudert, R. (2008). “Interatomic distances in pyrite-structure disulfides – a case for ellipsoidal modeling of sulfur ions” (PDF). Physica Status Solidi B. 245 (9): 1858–1864. Bibcode:2008PSSBR.245.1858B. doi:10.1002/pssb.200879532. S2CID 97824066. Archived (PDF) from the original on 2 May 2021. Retrieved 2 May 2021.
- ^ Birkholz, M. (2014). “Modeling the Shape of Ions in Pyrite-Type Crystals”. Crystals. 4 (3): 390–403. doi:10.3390/cryst4030390.
- ^ Staff (2007). “Small Miracles: Harnessing nanotechnology”. Oregon State University. Archived from the original on 21 May 2011. – describes the width of a human hair as 105 nm and 10 carbon atoms as spanning 1 nm.
- ^ Padilla, Michael J.; Miaoulis, Ioannis; Cyr, Martha (2002). Prentice Hall Science Explorer: Chemical Building Blocks. Upper Saddle River, New Jersey: Prentice-Hall, Inc. p. 32. ISBN 978-0-13-054091-1. OCLC 47925884.
There are 2,000,000,000,000,000,000,000 (that’s 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen.
- ^ “The Feynman Lectures on Physics Vol. I Ch. 1: Atoms in Motion”. Archived from the original on 30 July 2022. Retrieved 3 May 2022.
- ^ Jump up to:a b “Radioactivity”. Splung.com. Archived from the original on 4 December 2007. Retrieved 19 December 2007.
- ^ L’Annunziata, Michael F. (2003). Handbook of Radioactivity Analysis. Academic Press. pp. 3–56. ISBN 978-0-12-436603-9. OCLC 16212955.
- ^ Firestone, Richard B. (22 May 2000). “Radioactive Decay Modes”. Berkeley Laboratory. Archived from the original on 29 September 2006.
- ^ Hornak, J.P. (2006). “Chapter 3: Spin Physics”. The Basics of NMR. Rochester Institute of Technology. Archived from the original on 3 February 2007.
- ^ Jump up to:a b Schroeder, Paul A. (25 February 2000). “Magnetic Properties”. University of Georgia. Archived from the original on 29 April 2007.
- ^ Goebel, Greg (1 September 2007). “[4.3] Magnetic Properties of the Atom”. Elementary Quantum Physics. In The Public Domain website. Archived from the original on 29 June 2011.
- ^ Yarris, Lynn (Spring 1997). “Talking Pictures”. Berkeley Lab Research Review. Archived from the original on 13 January 2008.
- ^ Liang, Z.-P.; Haacke, E.M. (1999). Webster, J.G. (ed.). Encyclopedia of Electrical and Electronics Engineering: Magnetic Resonance Imaging. Vol. 2. John Wiley & Sons. pp. 412–426. ISBN 978-0-471-13946-1.
- ^ Zeghbroeck, Bart J. Van (1998). “Energy levels”. Shippensburg University. Archived from the original on 15 January 2005.
- ^ Fowles, Grant R. (1989). Introduction to Modern Optics. Courier Dover Publications. pp. 227–233. ISBN 978-0-486-65957-2. OCLC 18834711.
- ^ Martin, W.C.; Wiese, W.L. (May 2007). “Atomic Spectroscopy: A Compendium of Basic Ideas, Notation, Data, and Formulas”. National Institute of Standards and Technology. Archived from the original on 8 February 2007.
- ^ “Atomic Emission Spectra – Origin of Spectral Lines”. Avogadro Web Site. Archived from the original on 28 February 2006. Retrieved 10 August 2006.
- ^ Fitzpatrick, Richard (16 February 2007). “Fine structure”. University of Texas at Austin. Archived from the original on 27 September 2011.
- ^ Weiss, Michael (2001). “The Zeeman Effect”. University of California-Riverside. Archived from the original on 2 February 2008.
- ^ Beyer, H.F.; Shevelko, V.P. (2003). Introduction to the Physics of Highly Charged Ions. CRC Press. pp. 232–236. ISBN 978-0-7503-0481-8. OCLC 47150433.
- ^ Watkins, Thayer. “Coherence in Stimulated Emission”. San José State University. Archived from the original on 12 January 2008. Retrieved 23 December 2007.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”) (1997). Online corrected version: (2006–) “valence“. doi:10.1351/goldbook.V06588
- ^ Reusch, William (16 July 2007). “Virtual Textbook of Organic Chemistry”. Michigan State University. Archived from the original on 29 October 2007.
- ^ “Covalent bonding – Single bonds”. chemguide. 2000. Archived from the original on 1 November 2008.
- ^ Husted, Robert; et al. (11 December 2003). “Periodic Table of the Elements”. Los Alamos National Laboratory. Archived from the original on 10 January 2008.
- ^ Baum, Rudy (2003). “It’s Elemental: The Periodic Table”. Chemical & Engineering News. Archived from the original on 6 April 2011.
- ^ Goodstein, David L. (2002). States of Matter. Courier Dover Publications. pp. 436–438. ISBN 978-0-13-843557-8.
- ^ Brazhkin, Vadim V. (2006). “Metastable phases, phase transformations, and phase diagrams in physics and chemistry”. Physics-Uspekhi. 49 (7): 719–724. Bibcode:2006PhyU…49..719B. doi:10.1070/PU2006v049n07ABEH006013. S2CID 93168446.
- ^ Myers, Richard (2003). The Basics of Chemistry. Greenwood Press. p. 85. ISBN 978-0-313-31664-7. OCLC 50164580.
- ^ Staff (9 October 2001). “Bose–Einstein Condensate: A New Form of Matter”. National Institute of Standards and Technology. Archived from the original on 3 January 2008.
- ^ Colton, Imogen; Fyffe, Jeanette (3 February 1999). “Super Atoms from Bose–Einstein Condensation”. The University of Melbourne. Archived from the original on 29 August 2007.
- ^ Jacox, Marilyn; Gadzuk, J. William (November 1997). “Scanning Tunneling Microscope”. National Institute of Standards and Technology. Archived from the original on 7 January 2008.
- ^ “The Nobel Prize in Physics 1986”. The Nobel Foundation. Archived from the original on 17 September 2008. Retrieved 11 January 2008. In particular, see the Nobel lecture by G. Binnig and H. Rohrer.
- ^ Jakubowski, N.; Moens, Luc; Vanhaecke, Frank (1998). “Sector field mass spectrometers in ICP-MS”. Spectrochimica Acta Part B: Atomic Spectroscopy. 53 (13): 1739–1763. Bibcode:1998AcSpB..53.1739J. doi:10.1016/S0584-8547(98)00222-5.
- ^ Müller, Erwin W.; Panitz, John A.; McLane, S. Brooks (1968). “The Atom-Probe Field Ion Microscope”. Review of Scientific Instruments. 39 (1): 83–86. Bibcode:1968RScI…39…83M. doi:10.1063/1.1683116.
- ^ Lochner, Jim; Gibb, Meredith; Newman, Phil (30 April 2007). “What Do Spectra Tell Us?”. NASA/Goddard Space Flight Center. Archived from the original on 16 January 2008.
- ^ Winter, Mark (2007). “Helium”. WebElements. Archived from the original on 30 December 2007.
- ^ Hinshaw, Gary (10 February 2006). “What is the Universe Made Of?”. NASA/WMAP. Archived from the original on 31 December 2007.
- ^ Choppin, Gregory R.; Liljenzin, Jan-Olov; Rydberg, Jan (2001). Radiochemistry and Nuclear Chemistry. Elsevier. p. 441. ISBN 978-0-7506-7463-8. OCLC 162592180.
- ^ Davidsen, Arthur F. (1993). “Far-Ultraviolet Astronomy on the Astro-1 Space Shuttle Mission”. Science. 259 (5093): 327–334. Bibcode:1993Sci…259..327D. doi:10.1126/science.259.5093.327. PMID 17832344. S2CID 28201406.
- ^ Lequeux, James (2005). The Interstellar Medium. Springer. p. 4. ISBN 978-3-540-21326-0. OCLC 133157789.
- ^ Smith, Nigel (6 January 2000). “The search for dark matter”. Physics World. Archived from the original on 16 February 2008.
- ^ Croswell, Ken (1991). “Boron, bumps and the Big Bang: Was matter spread evenly when the Universe began? Perhaps not; the clues lie in the creation of the lighter elements such as boron and beryllium”. New Scientist (1794): 42. Archived from the original on 7 February 2008.
- ^ Copi, Craig J.; Schramm, DN; Turner, MS (1995). “Big-Bang Nucleosynthesis and the Baryon Density of the Universe”. Science (Submitted manuscript). 267 (5195): 192–199. arXiv:astro-ph/9407006. Bibcode:1995Sci…267..192C. doi:10.1126/science.7809624. PMID 7809624. S2CID 15613185. Archived from the original on 14 August 2019.
- ^ Hinshaw, Gary (15 December 2005). “Tests of the Big Bang: The Light Elements”. NASA/WMAP. Archived from the original on 17 January 2008.
- ^ Abbott, Brian (30 May 2007). “Microwave (WMAP) All-Sky Survey”. Hayden Planetarium. Archived from the original on 13 February 2013.
- ^ Hoyle, F. (1946). “The synthesis of the elements from hydrogen”. Monthly Notices of the Royal Astronomical Society. 106 (5): 343–383. Bibcode:1946MNRAS.106..343H. doi:10.1093/mnras/106.5.343.
- ^ Knauth, D.C.; Knauth, D.C.; Lambert, David L.; Crane, P. (2000). “Newly synthesized lithium in the interstellar medium”. Nature. 405 (6787): 656–658. Bibcode:2000Natur.405..656K. doi:10.1038/35015028. PMID 10864316. S2CID 4397202.
- ^ Mashnik, Stepan G. (2000). “On Solar System and Cosmic Rays Nucleosynthesis and Spallation Processes”. arXiv:astro-ph/0008382.
- ^ Kansas Geological Survey (4 May 2005). “Age of the Earth”. University of Kansas. Archived from the original on 5 July 2008.
- ^ Jump up to:a b Manuel (2001). Origin of Elements in the Solar System, pp. 40–430, 511–519
- ^ Dalrymple, G. Brent (2001). “The age of the Earth in the twentieth century: a problem (mostly) solved”. Geological Society, London, Special Publications. 190 (1): 205–221. Bibcode:2001GSLSP.190..205D. doi:10.1144/GSL.SP.2001.190.01.14. S2CID 130092094. Archived from the original on 11 November 2007.
- ^ Anderson, Don L.; Foulger, G.R.; Meibom, Anders (2 September 2006). “Helium: Fundamental models”. MantlePlumes.org. Archived from the original on 8 February 2007.
- ^ Pennicott, Katie (10 May 2001). “Carbon clock could show the wrong time”. PhysicsWeb. Archived from the original on 15 December 2007.
- ^ Yarris, Lynn (27 July 2001). “New Superheavy Elements 118 and 116 Discovered at Berkeley Lab”. Berkeley Lab. Archived from the original on 9 January 2008.
- ^ Diamond, H; et al. (1960). “Heavy Isotope Abundances in Mike Thermonuclear Device”. Physical Review. 119 (6): 2000–2004. Bibcode:1960PhRv..119.2000D. doi:10.1103/PhysRev.119.2000.
- ^ Poston, John W. Sr. (23 March 1998). “Do transuranic elements such as plutonium ever occur naturally?”. Scientific American. Archived from the original on 27 March 2015.
- ^ Keller, C. (1973). “Natural occurrence of lanthanides, actinides, and superheavy elements”. Chemiker Zeitung. 97 (10): 522–530. OSTI 4353086.
- ^ Zaider, Marco; Rossi, Harald H. (2001). Radiation Science for Physicians and Public Health Workers. Springer. p. 17. ISBN 978-0-306-46403-4. OCLC 44110319.
- ^ “Oklo Fossil Reactors”. Curtin University of Technology. Archived from the original on 18 December 2007. Retrieved 15 January 2008.
- ^ Weisenberger, Drew. “How many atoms are there in the world?”. Jefferson Lab. Archived from the original on 22 October 2007. Retrieved 16 January 2008.
- ^ Pidwirny, Michael. “Fundamentals of Physical Geography”. University of British Columbia Okanagan. Archived from the original on 21 January 2008. Retrieved 16 January 2008.
- ^ Anderson, Don L. (2002). “The inner inner core of Earth”. Proceedings of the National Academy of Sciences. 99 (22): 13966–13968. Bibcode:2002PNAS…9913966A. doi:10.1073/pnas.232565899. PMC 137819. PMID 12391308.
- ^ Pauling, Linus (1960). The Nature of the Chemical Bond. Cornell University Press. pp. 5–10. ISBN 978-0-8014-0333-0. OCLC 17518275.
- ^ Anonymous (2 October 2001). “Second postcard from the island of stability”. CERN Courier. Archived from the original on 3 February 2008.
- ^ Karpov, A. V.; Zagrebaev, V. I.; Palenzuela, Y. M.; et al. (2012). “Decay properties and stability of the heaviest elements” (PDF). International Journal of Modern Physics E. 21 (2): 1250013-1–1250013-20. Bibcode:2012IJMPE..2150013K. doi:10.1142/S0218301312500139. Archived (PDF) from the original on 3 December 2016. Retrieved 24 March 2020.
- ^ “Superheavy Element 114 Confirmed: A Stepping Stone to the Island of Stability”. Berkeley Lab. 2009. Archived from the original on 20 July 2019. Retrieved 24 March 2020.
- ^ Möller, P. (2016). “The limits of the nuclear chart set by fission and alpha decay” (PDF). EPJ Web of Conferences. 131: 03002-1–03002-8. Bibcode:2016EPJWC.13103002M. doi:10.1051/epjconf/201613103002. Archived (PDF) from the original on 11 March 2020. Retrieved 24 March 2020.
- ^ Koppes, Steve (1 March 1999). “Fermilab Physicists Find New Matter-Antimatter Asymmetry”. University of Chicago. Archived from the original on 19 July 2008.
- ^ Cromie, William J. (16 August 2001). “A lifetime of trillionths of a second: Scientists explore antimatter”. Harvard University Gazette. Archived from the original on 3 September 2006.
- ^ Hijmans, Tom W. (2002). “Particle physics: Cold antihydrogen”. Nature. 419 (6906): 439–440. Bibcode:2002Natur.419..439H. doi:10.1038/419439a. PMID 12368837.
- ^ Staff (30 October 2002). “Researchers ‘look inside’ antimatter”. BBC News. Archived from the original on 22 February 2007.
- ^ Barrett, Roger (1990). “The Strange World of the Exotic Atom”. New Scientist (1728): 77–115. Archived from the original on 21 December 2007.
- ^ Indelicato, Paul (2004). “Exotic Atoms”. Physica Scripta. T112 (1): 20–26. arXiv:physics/0409058. Bibcode:2004PhST..112…20I. doi:10.1238/Physica.Topical.112a00020. S2CID 11134265. Archived from the original on 4 November 2018.
- ^ Ripin, Barrett H. (July 1998). “Recent Experiments on Exotic Atoms”. American Physical Society. Archived from the original on 23 July 2012.



